当前位置:
X-MOL 学术
›
Appl. Clay. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
AFM measurements of Hofmeister effects on clay mineral particle interaction forces
Applied Clay Science ( IF 5.3 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.clay.2020.105443 Bo Feng , Hanyi Liu , Yingli Li , Xinmin Liu , Rui Tian , Rui Li , Hang Li
Applied Clay Science ( IF 5.3 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.clay.2020.105443 Bo Feng , Hanyi Liu , Yingli Li , Xinmin Liu , Rui Tian , Rui Li , Hang Li
|
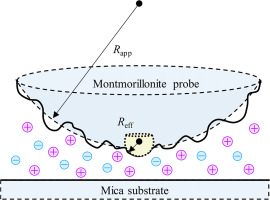
Abstract Interaction forces between clay minerals determine particle aggregation and dispersion of clay suspension. Strong Hofmeister effects have been found in surface reactions of clay mineral particles, clay aggregation kinetics, and clay aggregate stability. However, Hofmeister effects observed so far lack experimental evidence from direct measurements of clay mineral particle interaction forces. Through introducing a parameter of “effective radius” for clay mineral particle with a rough surface, AFM was successfully employed to determine the interaction forces between montmorillonite (with roughly surface) and mica (with smooth surface), and strong Hofmeister effects on particle interaction forces were directly observed. The directly measured repulsive forces between the two particles followed the sequence of Li+ > Na+ > K+ > Cs+, and the force strength in solution with 10−4 mol L−1 Li+ was about 3.6 times that in solution with 10−4 mol L−1 Cs+. Correspondingly, the determined average Stern potential of montmorillonite and mica decreased in the sequence of Li+ > Na+ > K+ > Cs+, and the Stern potential in solution with 10−4 mol L−1 Li+ was about 2.58 times that in solution with 10−4 mol L−1 Cs+. Analyses of AFM measurements showed that the cation surface adsorption energy determined the ability of cations to shield the electric field around clay mineral particles, which led to the Hofmeister sequence of the Stern potentials of clay mineral particle and the electrostatic repulsive forces between the two clay mineral particles.
中文翻译:

霍夫迈斯特效应对粘土矿物颗粒相互作用力的 AFM 测量
摘要 粘土矿物之间的相互作用力决定了粘土悬浮液的颗粒聚集和分散。在粘土矿物颗粒的表面反应、粘土聚集动力学和粘土聚集体稳定性中发现了强烈的霍夫迈斯特效应。然而,迄今为止观察到的霍夫迈斯特效应缺乏直接测量粘土矿物颗粒相互作用力的实验证据。通过引入粗糙表面粘土矿物颗粒的“有效半径”参数,成功地利用原子力显微镜确定蒙脱石(表面粗糙)和云母(表面光滑)之间的相互作用力,以及对颗粒相互作用力的强霍夫迈斯特效应被直接观察。直接测量的两个粒子之间的排斥力遵循 Li+ > Na+ > K+ > Cs+ 的顺序,10-4 mol L-1 Li+溶液的力强度约为10-4 mol L-1 Cs+溶液的3.6倍。相应地,蒙脱石和云母测定的平均 Stern 电位按 Li+ > Na+ > K+ > Cs+ 的顺序降低,10−4 mol L−1 Li+ 溶液中的 Stern 电位约为 10−4 溶液的 2.58 倍。 mol L−1 Cs+。AFM 测量分析表明,阳离子表面吸附能决定了阳离子屏蔽粘土矿物颗粒周围电场的能力,这导致了粘土矿物颗粒 Stern 电位的 Hofmeister 序列和两种粘土矿物之间的静电排斥力粒子。蒙脱石和云母测定的平均 Stern 电位按 Li+ > Na+ > K+ > Cs+ 的顺序降低,10-4 mol L-1 Li+ 溶液的 Stern 电位约为 10-4 mol L 溶液的 2.58 倍-1 Cs+。AFM 测量分析表明,阳离子表面吸附能决定了阳离子屏蔽粘土矿物颗粒周围电场的能力,这导致了粘土矿物颗粒 Stern 电位的 Hofmeister 序列和两种粘土矿物之间的静电排斥力粒子。蒙脱石和云母测定的平均 Stern 电位按 Li+ > Na+ > K+ > Cs+ 的顺序降低,10-4 mol L-1 Li+ 溶液的 Stern 电位约为 10-4 mol L 溶液的 2.58 倍-1 Cs+。AFM 测量分析表明,阳离子表面吸附能决定了阳离子屏蔽粘土矿物颗粒周围电场的能力,这导致了粘土矿物颗粒 Stern 电位的 Hofmeister 序列和两种粘土矿物之间的静电排斥力粒子。
更新日期:2020-03-01
中文翻译:

霍夫迈斯特效应对粘土矿物颗粒相互作用力的 AFM 测量
摘要 粘土矿物之间的相互作用力决定了粘土悬浮液的颗粒聚集和分散。在粘土矿物颗粒的表面反应、粘土聚集动力学和粘土聚集体稳定性中发现了强烈的霍夫迈斯特效应。然而,迄今为止观察到的霍夫迈斯特效应缺乏直接测量粘土矿物颗粒相互作用力的实验证据。通过引入粗糙表面粘土矿物颗粒的“有效半径”参数,成功地利用原子力显微镜确定蒙脱石(表面粗糙)和云母(表面光滑)之间的相互作用力,以及对颗粒相互作用力的强霍夫迈斯特效应被直接观察。直接测量的两个粒子之间的排斥力遵循 Li+ > Na+ > K+ > Cs+ 的顺序,10-4 mol L-1 Li+溶液的力强度约为10-4 mol L-1 Cs+溶液的3.6倍。相应地,蒙脱石和云母测定的平均 Stern 电位按 Li+ > Na+ > K+ > Cs+ 的顺序降低,10−4 mol L−1 Li+ 溶液中的 Stern 电位约为 10−4 溶液的 2.58 倍。 mol L−1 Cs+。AFM 测量分析表明,阳离子表面吸附能决定了阳离子屏蔽粘土矿物颗粒周围电场的能力,这导致了粘土矿物颗粒 Stern 电位的 Hofmeister 序列和两种粘土矿物之间的静电排斥力粒子。蒙脱石和云母测定的平均 Stern 电位按 Li+ > Na+ > K+ > Cs+ 的顺序降低,10-4 mol L-1 Li+ 溶液的 Stern 电位约为 10-4 mol L 溶液的 2.58 倍-1 Cs+。AFM 测量分析表明,阳离子表面吸附能决定了阳离子屏蔽粘土矿物颗粒周围电场的能力,这导致了粘土矿物颗粒 Stern 电位的 Hofmeister 序列和两种粘土矿物之间的静电排斥力粒子。蒙脱石和云母测定的平均 Stern 电位按 Li+ > Na+ > K+ > Cs+ 的顺序降低,10-4 mol L-1 Li+ 溶液的 Stern 电位约为 10-4 mol L 溶液的 2.58 倍-1 Cs+。AFM 测量分析表明,阳离子表面吸附能决定了阳离子屏蔽粘土矿物颗粒周围电场的能力,这导致了粘土矿物颗粒 Stern 电位的 Hofmeister 序列和两种粘土矿物之间的静电排斥力粒子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号