Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.molliq.2020.112531 Stephanie MacDonald , Shannon MacLennan , D. Gerrard Marangoni
|
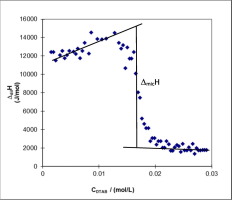
The thermodynamic parameters of surfactant/alcohol mixed micelles were determined in mixtures of sodium dodecyl sulfate (SDS) and dodecyltrimethylammonium bromide (DTAB) in a series of solvents composed of varying concentrations of n-propanol (C3OH), n-butanol (C4OH), and n-pentanol (C5OH). Conductivity studies were used to determine CMC values and the degrees of counterion binding. Isoperibol solution calorimetry was used to directly determine CMC values and enthalpy of micellization (ΔmicH). From these experimentally determined values, the Gibbs free energy and entropy of micellization were calculated as a function of temperature and alcohol concentration. In general, CMC values decreased with increasing alcohol carbon chain length and concentration but increased with temperature. The effects of temperature, alcohol carbon chain length, and alcohol concentration on the energetics of these mixed micellar systems are explained in terms of the relative contributions to ΔmicH by alkyl chain interactions, hydrophobic effects, and electrostatic contributions.
中文翻译:

量热法测定醇表面活性剂混合胶束形成的热力学:温度和浓度的影响
在十二烷基硫酸钠(SDS)和十二烷基三甲基溴化铵(DTAB)的混合物中,在一系列由浓度不同的正丙醇(C 3 OH),正丁醇(C)组成的溶剂中确定表面活性剂/醇混合胶束的热力学参数4 OH)和正戊醇(C 5 OH)。电导率研究用于确定CMC值和抗衡离子结合程度。等温环境溶解量热法用于直接确定CMC值和胶束化的焓(Δ麦克风H)。从这些实验确定的值,吉布斯自由能和胶束化熵被计算为温度和酒精浓度的函数。通常,CMC值随醇碳链长度和浓度的增加而降低,但随温度升高而增加。温度,醇碳链长度和醇浓度对这些混合胶束系统的能级的影响是通过烷基链相互作用对Δmic H的相对贡献,疏水作用和静电作用来解释的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号