Nature Nanotechnology ( IF 38.1 ) Pub Date : 2020-01-20 , DOI: 10.1038/s41565-019-0620-x Benjamin Doppagne 1 , Tomáš Neuman 2 , Ruben Soria-Martinez 1 , Luis E Parra López 1 , Hervé Bulou 1 , Michelangelo Romeo 1 , Stéphane Berciaud 1 , Fabrice Scheurer 1 , Javier Aizpurua 2 , Guillaume Schull 1
|
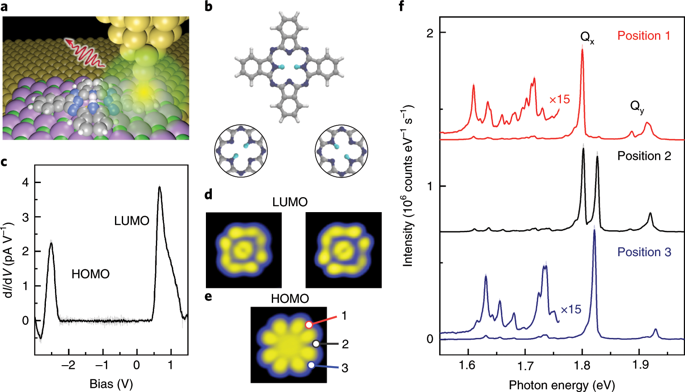
Tautomerization, the interconversion between two constitutional molecular isomers, is ubiquitous in nature1, plays a major role in chemistry2 and is perceived as an ideal switch function for emerging molecular-scale devices3. Within free-base porphyrin4, porphycene5 or phthalocyanine6, this process involves the concerted or sequential hopping of the two inner hydrogen atoms between equivalent nitrogen sites of the molecular cavity. Electronic and vibronic changes6 that result from this NH tautomerization, as well as details of the switching mechanism, were extensively studied with optical spectroscopies, even with single-molecule sensitivity7. The influence of atomic-scale variations of the molecular environment and submolecular spatial resolution of the tautomerization could only be investigated using scanning probe microscopes3,8,9,10,11, at the expense of detailed information provided by optical spectroscopies. Here, we combine these two approaches, scanning tunnelling microscopy (STM) and fluorescence spectroscopy12,13,14,15, to study the tautomerization within individual free-base phthalocyanine (H2Pc) molecules deposited on a NaCl-covered Ag(111) single-crystal surface. STM-induced fluorescence (STM-F) spectra exhibit duplicate features that we assign to the emission of the two molecular tautomers. We support this interpretation by comparing hyper-resolved fluorescence maps15,16,17,18(HRFMs) of the different spectral contributions with simulations that account for the interaction between molecular excitons and picocavity plasmons19. We identify the orientation of the molecular optical dipoles, determine the vibronic fingerprint of the tautomers and probe the influence of minute changes in their atomic-scale environment. Time-correlated fluorescence measurements allow us to monitor the tautomerization events and to associate the proton dynamics to a switching two-level system. Finally, optical spectra acquired with the tip located at a nanometre-scale distance from the molecule show that the tautomerization reaction occurs even when the tunnelling current does not pass through the molecule. Together with other observations, this remote excitation indicates that the excited state of the molecule is involved in the tautomerization reaction path.
中文翻译:

通过空间和时间分辨荧光光谱进行单分子互变异构跟踪
互变异构是两种结构分子异构体之间的相互转换,在自然界中无处不在1,在化学中发挥着重要作用2并被认为是新兴分子尺度器件3的理想开关功能。在游离碱卟啉4、卟啉5或酞菁6 中,该过程涉及分子腔的等效氮位点之间的两个内部氢原子的协同或连续跳跃。由这种 NH 互变异构化产生的电子和振动变化6以及开关机制的细节,都通过光谱学进行了广泛研究,即使是单分子灵敏度7. 分子环境的原子尺度变化和互变异构化的亚分子空间分辨率的影响只能使用扫描探针显微镜3,8,9,10,11进行研究,代价是光学光谱提供的详细信息。在这里,我们结合这两种方法,扫描隧道显微镜 (STM) 和荧光光谱12,13,14,15 ,研究沉积在 NaCl 覆盖的 Ag(111) 上的单个游离碱酞菁 (H 2 Pc) 分子内的互变异构化) 单晶表面。STM 诱导荧光 (STM-F) 光谱表现出我们分配给两个分子互变异构体发射的重复特征。我们通过比较超分辨率荧光图来支持这种解释15,16,17,18 (HRFM) 的不同光谱贡献与模拟说明分子激子和微腔等离子体之间的相互作用19. 我们确定了分子光学偶极子的方向,确定了互变异构体的振动指纹,并探索了它们原子尺度环境中微小变化的影响。时间相关的荧光测量使我们能够监测互变异构事件并将质子动力学与切换的两级系统相关联。最后,使用位于距分子纳米级距离的尖端获得的光谱表明,即使隧道电流不通过分子,也会发生互变异构反应。与其他观察结果一起,这种远程激发表明分子的激发态参与了互变异构反应路径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号