Nature Immunology ( IF 27.7 ) Pub Date : 2020-01-20 , DOI: 10.1038/s41590-019-0582-z Eniko Sajti 1 , Verena M Link 2, 3 , Zhengyu Ouyang 2 , Nathanael J Spann 2 , Emma Westin 2 , Casey E Romanoski 2, 4 , Gregory J Fonseca 2, 5 , Lawrence S Prince 1 , Christopher K Glass 2, 6
|
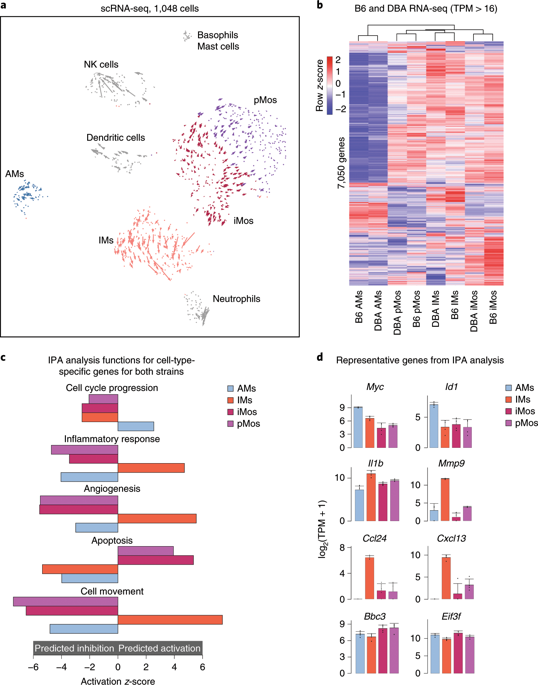
The lung is inhabited by resident alveolar and interstitial macrophages as well as monocytic cells that survey lung tissues. Each cell type plays distinct functional roles under homeostatic and inflammatory conditions, but mechanisms establishing their molecular identities and functional potential remain poorly understood. In the present study, systematic evaluation of transcriptomes and open chromatin of alveolar macrophages (AMs), interstitial macrophages (IMs) and lung monocytes from two mouse strains enabled inference of common and cell-specific transcriptional regulators. We provide evidence that these factors drive selection of regulatory landscapes that specify distinct phenotypes of AMs and IMs and entrain qualitatively different responses to toll-like receptor 4 signaling in vivo. These studies reveal a striking divergence in a fundamental innate immune response pathway in AMs and establish a framework for further understanding macrophage diversity in the lung.
中文翻译:

肺髓样多样性的转录组学和表观遗传机制
肺由常驻肺泡和间质巨噬细胞以及调查肺组织的单核细胞居住。每种细胞类型在稳态和炎症条件下发挥不同的功能作用,但建立其分子身份和功能潜力的机制仍然知之甚少。在本研究中,对来自两种小鼠品系的肺泡巨噬细胞 (AMs)、间质巨噬细胞 (IMs) 和肺单核细胞的转录组和开放染色质进行系统评估,可以推断出常见的和细胞特异性转录调节因子。我们提供的证据表明,这些因素推动了监管景观的选择,这些景观指定了 AM 和 IM 的不同表型,并在体内对 toll 样受体 4 信号传导产生了质的不同反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号