Nature Chemical Biology ( IF 12.9 ) Pub Date : 2020-01-20 , DOI: 10.1038/s41589-019-0457-5 Shunsuke Imai 1 , Tomoki Yokomizo 1 , Yutaka Kofuku 1 , Yutaro Shiraishi 1 , Takumi Ueda 1 , Ichio Shimada 1
|
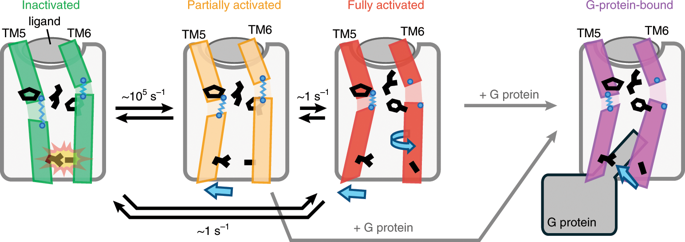
G-protein-coupled receptors (GPCRs) are seven-transmembrane proteins mediating cellular signals in response to extracellular stimuli. Although three-dimensional structures showcase snapshots that can be sampled in the process and nuclear magnetic resonance detects conformational equilibria, the mechanism by which agonist-activated GPCRs interact with various effectors remains elusive. Here, we used paramagnetic nuclear magnetic resonance for leucine amide resonances to visualize the structure of β2-adrenoreceptor in the full agonist-bound state, without thermostabilizing mutations abolishing its activity. The structure exhibited a unique orientation of the intracellular half of the transmembrane helix 6, forming a cluster of G-protein-interacting residues. Furthermore, analyses of efficacy-dependent chemical shifts of the residues near the pivotal PIF microswitch identified an equilibrium among three conformations, including one responsible for the varied signal level in each ligand-bound state. Together, these results provide a structural basis for the dynamic activation of GPCRs and shed light on GPCR-mediated signal transduction.
中文翻译:

β2-肾上腺素受体配体依赖性激活的结构平衡
G 蛋白偶联受体 (GPCR) 是七跨膜蛋白,可介导响应细胞外刺激的细胞信号。尽管 3D 结构展示了可以在该过程中采样的快照,并且核磁共振检测了构象平衡,但激动剂激活的 GPCR 与各种效应器相互作用的机制仍然难以捉摸。在这里,我们使用顺磁核磁共振进行亮氨酸酰胺共振来可视化 β 2的结构-完全激动剂结合状态的肾上腺素受体,没有消除其活性的热稳定突变。该结构表现出跨膜螺旋 6 的细胞内一半的独特方向,形成一簇 G 蛋白相互作用残基。此外,对关键 PIF 微开关附近残基的功效依赖性化学位移的分析确定了三种构象之间的平衡,其中一种构象负责每种配体结合状态下的不同信号水平。总之,这些结果为 GPCR 的动态激活提供了结构基础,并阐明了 GPCR 介导的信号转导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号