Nature Chemical Biology ( IF 14.8 ) Pub Date : 2020-01-20 , DOI: 10.1038/s41589-019-0455-7 Lingfeng Chen 1, 2, 3 , William M Marsiglia 4 , Huaibin Chen 2 , Joseph Katigbak 4 , Hediye Erdjument-Bromage 5 , David J Kemble 6 , Lili Fu 2, 3 , Jinghong Ma 2 , Gongqin Sun 6 , Yingkai Zhang 4 , Guang Liang 1, 3 , Thomas A Neubert 5 , Xiaokun Li 3 , Nathaniel J Traaseth 4 , Moosa Mohammadi 2
|
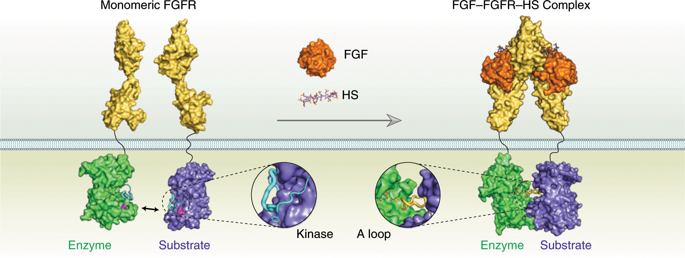
A long-standing mystery shrouds the mechanism by which catalytically repressed receptor tyrosine kinase domains accomplish transphosphorylation of activation loop (A-loop) tyrosines. Here we show that this reaction proceeds via an asymmetric complex that is thermodynamically disadvantaged because of an electrostatic repulsion between enzyme and substrate kinases. Under physiological conditions, the energetic gain resulting from ligand-induced dimerization of extracellular domains overcomes this opposing clash, stabilizing the A-loop-transphosphorylating dimer. A unique pathogenic fibroblast growth factor receptor gain-of-function mutation promotes formation of the complex responsible for phosphorylation of A-loop tyrosines by eliminating this repulsive force. We show that asymmetric complex formation induces a more phosphorylatable A-loop conformation in the substrate kinase, which in turn promotes the active state of the enzyme kinase. This explains how quantitative differences in the stability of ligand-induced extracellular dimerization promotes formation of the intracellular A-loop-transphosphorylating asymmetric complex to varying extents, thereby modulating intracellular kinase activity and signaling intensity.
中文翻译:

受体酪氨酸激酶A-环酪氨酸转磷酸化的分子基础
一个长期存在的谜团笼罩着催化抑制受体酪氨酸激酶结构域完成激活环(A-环)酪氨酸转磷酸化的机制。在这里,我们表明该反应通过不对称复合物进行,由于酶和底物激酶之间的静电排斥,该复合物在热力学上处于不利地位。在生理条件下,由配体诱导的细胞外结构域二聚化产生的能量增益克服了这种相反的冲突,稳定了 A 环转磷酸化二聚体。一种独特的致病性成纤维细胞生长因子受体功能获得性突变通过消除这种排斥力促进了负责 A 环酪氨酸磷酸化的复合物的形成。我们表明不对称复合物的形成在底物激酶中诱导了更可磷酸化的 A 环构象,这反过来又促进了酶激酶的活性状态。这解释了配体诱导的细胞外二聚化稳定性的定量差异如何在不同程度上促进细胞内 A-环转磷酸化不对称复合物的形成,从而调节细胞内激酶活性和信号强度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号