Nature Chemistry ( IF 19.2 ) Pub Date : 2020-01-20 , DOI: 10.1038/s41557-019-0407-6 Jian Li 1 , Fuzhuo Li 1 , Emma King-Smith 1 , Hans Renata 1
|
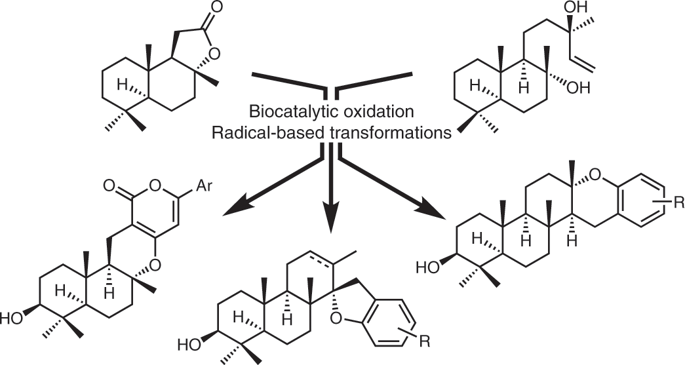
Meroterpenoids are natural products of hybrid biosynthetic origins—derived from both terpenoid and polyketide pathways—with a wealth of biological activities. Given their therapeutic potential, a general strategy to access these natural products in a concise and divergent fashion is highly desirable. Here, we report a modular synthesis of a suite of oxidized meroterpenoids using a hybrid synthetic strategy that is designed to harness the power of both biocatalytic and radical-based retrosynthetic logic. This strategy enables direct introduction of key hydroxyl groups and rapid construction of key bonds and stereocentres, facilitating the development of a concise route (7–12 steps from commercial materials) to eight oxidized meroterpenoids from two common molecular scaffolds. This work lays the foundation for rapid access to a wide range of oxidized meroterpenoids through the use of similar hybrid strategy that combines two synthetic approaches.
中文翻译:

结合化学酶和基于自由基的逆合成逻辑,用于快速和模块化合成氧化类萜类化合物
Meroterpenoids 是混合生物合成来源的天然产物 - 源自萜类化合物和聚酮化合物途径 - 具有丰富的生物活性。鉴于它们的治疗潜力,以简洁和不同的方式获取这些天然产物的一般策略是非常可取的。在这里,我们报告了使用混合合成策略对一组氧化类萜的模块化合成,该策略旨在利用生物催化和基于自由基的逆合成逻辑的力量。该策略能够直接引入关键羟基并快速构建关键键和立体中心,从而促进从两种常见的分子支架开发出一条简洁的路线(从商业材料到 7-12 步)到八种氧化的类二萜。











































 京公网安备 11010802027423号
京公网安备 11010802027423号