Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
TL1A/TNFR2-mediated mitochondrial dysfunction of fibroblast-like synoviocytes increases inflammatory response in patients with rheumatoid arthritis via reactive oxygen species generation.
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-01-17 , DOI: 10.1111/febs.15181 Mahmoud Al-Azab 1, 2 , Eskandar Qaed 3 , Xunli Ouyang 1 , Abdalkhalig Elkhider 1 , Williams Walana 1 , Han Li 1 , Weiping Li 1 , Yawei Tang 1 , Salah Adlat 4 , Jing Wei 1 , Bing Wang 1 , Xia Li 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-01-17 , DOI: 10.1111/febs.15181 Mahmoud Al-Azab 1, 2 , Eskandar Qaed 3 , Xunli Ouyang 1 , Abdalkhalig Elkhider 1 , Williams Walana 1 , Han Li 1 , Weiping Li 1 , Yawei Tang 1 , Salah Adlat 4 , Jing Wei 1 , Bing Wang 1 , Xia Li 1
Affiliation
|
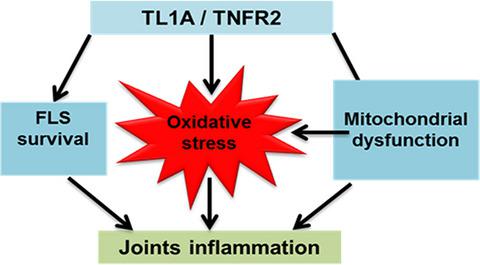
Rheumatoid arthritis (RA) is the major autoimmune destructive disease of joints with a complicated pathogenesis. The contribution of tumor necrosis factor‐like ligand 1A (TL1A) in RA pathogenesis, especially on fibroblast‐like synoviocytes (FLS), has been suggested clinically. The present study investigated the role of TL1A in mitochondrial dysfunction, induced oxidative stress in mitochondria, apoptosis resistance and the inflammatory response in FLS obtained from RA patients (RA‐FLS). RA‐FLS were incubated with TL1A and tumor necrosis factor receptor 2 (TNFR2) antagonist. Respiratory function, mitochondrial membrane potential and respiration associated genes of mitochondria were measured in both TL1A stimulated and non‐stimulated RA‐FLS. Additionally, the effects of TL1A on reactive oxygen species (ROS) production in mitochondria, apoptosis and the inflammatory response in RA‐FLS were also assessed. The role of TL1A in association between ROS generation, especially mitochondrial type and the inflammatory response, was evaluated by measuring inflammation‐related cytokines and signaling pathways using ROS inhibitors, diphenyleneiodonium chloride and Mito‐TEMPO (Sigma‐Aldrich, Miamisburg, OH, USA). We found that TL1A induced mitochondrial dysfunction by weakening mitochondrial respiration and membrane potential, which was blocked by a TNFR2 antagonist. Increased ROS synthesis in impaired mitochondria was observed with MitoSOX (Invitrogen, CA, USA) immunofluorescence staining in TL1A‐stimulated RA‐FLS but inhibited by a TNFR2 antagonist. TL1A influenced apoptosis resistance and inflammatory mediators via TNFR2. Inhibition of mitochondria‐derived ROS compromised the production of inflammatory factors in TL1A‐stimulated RA‐FLS, suggesting that mitochondrial dysfunction mediated by the TL1A/TNFR2 axis might amplify the inflammatory response via regulation of mitochondria‐derived ROS generation. Collectively, our results reveal that TL1A might be involved in making FLS more aggressive in RA pathogenesis via cell respiration interruption.
中文翻译:

TL1A / TNFR2介导的成纤维细胞样滑膜细胞线粒体功能障碍会通过活性氧的产生增加类风湿关节炎患者的炎症反应。
类风湿关节炎(RA)是一种主要的自身免疫性破坏性关节疾病,发病机理复杂。临床上已提出肿瘤坏死因子样配体1A(TL1A)在RA发病机制中的作用,特别是在成纤维样滑膜细胞(FLS)上。本研究调查了TL1A在线粒体功能障碍,线粒体中的氧化应激,细胞凋亡抗性以及从RA患者(FL-FLS)获得的FLS中的炎症反应中的作用。RA‐FLS与TL1A和肿瘤坏死因子受体2(TNFR2)拮抗剂孵育。在TL1A刺激和未刺激的RA-FLS中均测量了呼吸功能,线粒体膜电位和线粒体的呼吸相关基因。此外,TL1A对线粒体中活性氧(ROS)产生的影响,还评估了RA-FLS中的细胞凋亡和炎症反应。TL1A在ROS产生,特别是线粒体类型与炎症反应之间的关联中的作用是通过使用ROS抑制剂,二苯基碘化铵氯化物和Mito-TEMPO(Sigma-Aldrich,Miamisburg,OH,美国)测量炎症相关的细胞因子和信号通路来评估的。我们发现TL1A通过削弱线粒体呼吸和膜电位来诱导线粒体功能障碍,而后者被TNFR2拮抗剂阻断。在TL1A刺激的RA-FLS中用MitoSOX(Invitrogen,CA,USA)免疫荧光染色观察到,线粒体受损时ROS合成增加,但被TNFR2拮抗剂抑制。TL1A通过TNFR2影响细胞凋亡抗性和炎症介质。抑制线粒体来源的ROS会损害TL1A刺激的RA-FLS中炎症因子的产生,这表明由TL1A / TNFR2轴介导的线粒体功能障碍可能通过调节线粒体来源的ROS产生来放大炎症反应。总体而言,我们的结果表明TL1A可能参与通过细胞呼吸中断使FLS在RA发病机制中更具侵略性。
更新日期:2020-01-17
中文翻译:

TL1A / TNFR2介导的成纤维细胞样滑膜细胞线粒体功能障碍会通过活性氧的产生增加类风湿关节炎患者的炎症反应。
类风湿关节炎(RA)是一种主要的自身免疫性破坏性关节疾病,发病机理复杂。临床上已提出肿瘤坏死因子样配体1A(TL1A)在RA发病机制中的作用,特别是在成纤维样滑膜细胞(FLS)上。本研究调查了TL1A在线粒体功能障碍,线粒体中的氧化应激,细胞凋亡抗性以及从RA患者(FL-FLS)获得的FLS中的炎症反应中的作用。RA‐FLS与TL1A和肿瘤坏死因子受体2(TNFR2)拮抗剂孵育。在TL1A刺激和未刺激的RA-FLS中均测量了呼吸功能,线粒体膜电位和线粒体的呼吸相关基因。此外,TL1A对线粒体中活性氧(ROS)产生的影响,还评估了RA-FLS中的细胞凋亡和炎症反应。TL1A在ROS产生,特别是线粒体类型与炎症反应之间的关联中的作用是通过使用ROS抑制剂,二苯基碘化铵氯化物和Mito-TEMPO(Sigma-Aldrich,Miamisburg,OH,美国)测量炎症相关的细胞因子和信号通路来评估的。我们发现TL1A通过削弱线粒体呼吸和膜电位来诱导线粒体功能障碍,而后者被TNFR2拮抗剂阻断。在TL1A刺激的RA-FLS中用MitoSOX(Invitrogen,CA,USA)免疫荧光染色观察到,线粒体受损时ROS合成增加,但被TNFR2拮抗剂抑制。TL1A通过TNFR2影响细胞凋亡抗性和炎症介质。抑制线粒体来源的ROS会损害TL1A刺激的RA-FLS中炎症因子的产生,这表明由TL1A / TNFR2轴介导的线粒体功能障碍可能通过调节线粒体来源的ROS产生来放大炎症反应。总体而言,我们的结果表明TL1A可能参与通过细胞呼吸中断使FLS在RA发病机制中更具侵略性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号