Molecular Catalysis ( IF 3.9 ) Pub Date : 2020-01-20 , DOI: 10.1016/j.mcat.2020.110765 Hua Li , Yiying Yang , Zhe Han , Chengbu Liu , Dongju Zhang
|
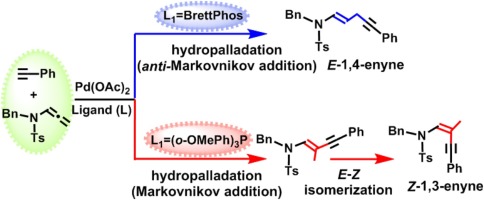
Density functional theory (DFT) calculations show that regardless of using BrettPhos or (o-OMePh)3P ligand, the Pd-catalyzed synthesis of both 1,3- and 1,4-enynes from hydroalkynylations of allenamides proceeds through the hydropalladation mechanism, rather than the carbopalladation mechanism, as proposed for the formation of 1,4-enyne in the experimental study. BrettPhos ligand favors the formation of 1,4-enyne through anti-Markovnikov addition of PdH bond to the C
C double bond, while (o-OMePh)3P ligand leads to the 1,3-enyne through the Markovnikov addition. The palladium catalysis also promotes the isomerization of E-1,3-enyne to its Z-isomer, rationalizing the observed stereoselectivity.
中文翻译:

由烯丙基酰胺的配体控制的区域发散性氢炔化作用,Pd催化立体选择性合成Z -1,3-和E -1,4-炔烃的计算机理研究
密度泛函理论(DFT)计算表明,无论使用BrettPhos或(o -OMePh)3 P配体,丙二烯酰胺的加氢羰基化反应都可以通过钯催化钯缩合法合成1,3-和1,4-炔炔,而不是像在实验研究中建议的形成1,4-烯炔那样的碳pal触滑机制。BrettPhos配体通过将Pd H键反Markovnikov加到C
C双键上而促进1,4-烯炔的形成,而(o -OMePh)3 P配体通过Markovnikov加成导致1,3- enyne的形成。钯催化还促进了E -1,3-烯炔的异构化为其Z-异构体,合理化观察到的立体选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号