当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal Structure of the Ryanodine Receptor SPRY2 Domain from the Diamondback Moth Provides Insights into the Development of Novel Insecticides.
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-01-17 , DOI: 10.1021/acs.jafc.9b08151 Yuanyuan Zhou,Dan Ma,Lianyun Lin,Minsheng You,Zhiguang Yuchi,Shijun You
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-01-17 , DOI: 10.1021/acs.jafc.9b08151 Yuanyuan Zhou,Dan Ma,Lianyun Lin,Minsheng You,Zhiguang Yuchi,Shijun You
|
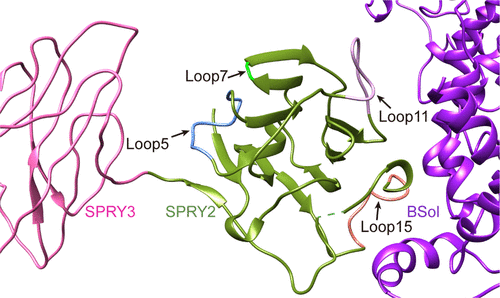
Diamide insecticides targeting ryanodine receptors (RyRs) are a major class of pesticides used to control a wide range of agricultural pests, but their efficacies have been reduced dramatically by the recent emergence of resistance mutations. There is a pressing need to develop novel insecticides, targeting distinct and novel binding sites within insect RyRs to overcome the resistance crisis; however, the limited structural information on insect RyRs is a major roadblock to our understanding of their molecular mechanisms. Here, we report the crystal structure of the RyR SPRY2 domain from the diamondback moth (DBM), Plutella xylostella, a destructive agricultural pest worldwide that has developed resistance to all classes of insecticide at 2.06 Å resolution. The overall fold of DBM SPRY2 is similar to its mammalian homolog, but it shows distinct conformations in several loops. Docking it into the recently published cryo-electron microscope structure of the full-length RyR reveals that two insect-specific loops interact with the BSol domain from the neighboring subunit. The SPRY2-BSol interface will change the conformation upon channel gating, indicating that it might be a potential targeting site for insect-specific insecticides. Interestingly, several previously identified disease-causing mutations also lie in the same interface, implying that this interface is important for channel gating. Another insect-specific loop located in the SPRY2-SPRY3 interface might indirectly affect another gating interface between SPRY3 and Repeat34.
中文翻译:

小菜蛾的赖安odine受体SPRY2域的晶体结构为新型杀虫剂的发展提供了见识。
靶向ryanodine受体(RyRs)的二酰胺类杀虫剂是一类主要的杀虫剂,可用于控制多种农业害虫,但是由于最近出现的抗性突变,其功效大大降低。迫切需要开发新的杀虫剂,以昆虫RyRs内不同且新颖的结合位点为目标,以克服抗药性危机。然而,关于昆虫RyRs的结构信息有限,这是我们了解其分子机理的主要障碍。在这里,我们报道了小菜蛾(Plutella xylostella)小菜蛾(Plutella xylostella)中RyR SPRY2域的晶体结构,该小菜蛾是全球范围内的破坏性农业害虫,已经对所有种类的杀虫剂产生了2.06Å的抗性。DBM SPRY2的整体折叠与其哺乳动物同源物相似,但它在几个循环中显示出截然不同的构象。将其对接到最近发布的全长RyR的低温电子显微镜结构中,发现两个昆虫特异性环与相邻亚基的BSol域相互作用。SPRY2-BSol界面在通道门控时会改变构象,表明它可能是昆虫特异性杀虫剂的潜在靶向位点。有趣的是,几个先前确定的致病突变也位于同一接口中,这表明该接口对于通道门控非常重要。位于SPRY2-SPRY3接口中的另一个昆虫特异性回路可能会间接影响SPRY3和Repeat34之间的另一个门控接口。将其对接到最近发布的全长RyR的低温电子显微镜结构中,发现两个昆虫特异性环与相邻亚基的BSol域相互作用。SPRY2-BSol界面在通道门控时会改变构象,表明它可能是昆虫特异性杀虫剂的潜在靶向位点。有趣的是,几个先前确定的致病突变也位于同一接口中,这表明该接口对于通道门控非常重要。位于SPRY2-SPRY3接口中的另一个昆虫特异性回路可能会间接影响SPRY3和Repeat34之间的另一个门控接口。将其对接到最近发布的全长RyR的低温电子显微镜结构中,发现两个昆虫特异性环与相邻亚基的BSol域相互作用。SPRY2-BSol界面将在通道门控时改变构象,表明它可能是昆虫特异性杀虫剂的潜在靶向位点。有趣的是,几个先前确定的致病突变也位于同一接口中,这表明该接口对于通道门控非常重要。位于SPRY2-SPRY3接口中的另一个昆虫特异性回路可能会间接影响SPRY3和Repeat34之间的另一个门控接口。表明它可能是昆虫特异性杀虫剂的潜在靶点。有趣的是,几个先前确定的致病突变也位于同一接口中,这表明该接口对于通道门控非常重要。位于SPRY2-SPRY3接口中的另一个昆虫特异性回路可能会间接影响SPRY3和Repeat34之间的另一个门控接口。表明它可能是昆虫特异性杀虫剂的潜在靶点。有趣的是,几个先前确定的致病突变也位于同一接口中,这表明该接口对于通道门控非常重要。位于SPRY2-SPRY3接口中的另一个昆虫特异性回路可能会间接影响SPRY3和Repeat34之间的另一个门控接口。
更新日期:2020-02-03
中文翻译:

小菜蛾的赖安odine受体SPRY2域的晶体结构为新型杀虫剂的发展提供了见识。
靶向ryanodine受体(RyRs)的二酰胺类杀虫剂是一类主要的杀虫剂,可用于控制多种农业害虫,但是由于最近出现的抗性突变,其功效大大降低。迫切需要开发新的杀虫剂,以昆虫RyRs内不同且新颖的结合位点为目标,以克服抗药性危机。然而,关于昆虫RyRs的结构信息有限,这是我们了解其分子机理的主要障碍。在这里,我们报道了小菜蛾(Plutella xylostella)小菜蛾(Plutella xylostella)中RyR SPRY2域的晶体结构,该小菜蛾是全球范围内的破坏性农业害虫,已经对所有种类的杀虫剂产生了2.06Å的抗性。DBM SPRY2的整体折叠与其哺乳动物同源物相似,但它在几个循环中显示出截然不同的构象。将其对接到最近发布的全长RyR的低温电子显微镜结构中,发现两个昆虫特异性环与相邻亚基的BSol域相互作用。SPRY2-BSol界面在通道门控时会改变构象,表明它可能是昆虫特异性杀虫剂的潜在靶向位点。有趣的是,几个先前确定的致病突变也位于同一接口中,这表明该接口对于通道门控非常重要。位于SPRY2-SPRY3接口中的另一个昆虫特异性回路可能会间接影响SPRY3和Repeat34之间的另一个门控接口。将其对接到最近发布的全长RyR的低温电子显微镜结构中,发现两个昆虫特异性环与相邻亚基的BSol域相互作用。SPRY2-BSol界面在通道门控时会改变构象,表明它可能是昆虫特异性杀虫剂的潜在靶向位点。有趣的是,几个先前确定的致病突变也位于同一接口中,这表明该接口对于通道门控非常重要。位于SPRY2-SPRY3接口中的另一个昆虫特异性回路可能会间接影响SPRY3和Repeat34之间的另一个门控接口。将其对接到最近发布的全长RyR的低温电子显微镜结构中,发现两个昆虫特异性环与相邻亚基的BSol域相互作用。SPRY2-BSol界面将在通道门控时改变构象,表明它可能是昆虫特异性杀虫剂的潜在靶向位点。有趣的是,几个先前确定的致病突变也位于同一接口中,这表明该接口对于通道门控非常重要。位于SPRY2-SPRY3接口中的另一个昆虫特异性回路可能会间接影响SPRY3和Repeat34之间的另一个门控接口。表明它可能是昆虫特异性杀虫剂的潜在靶点。有趣的是,几个先前确定的致病突变也位于同一接口中,这表明该接口对于通道门控非常重要。位于SPRY2-SPRY3接口中的另一个昆虫特异性回路可能会间接影响SPRY3和Repeat34之间的另一个门控接口。表明它可能是昆虫特异性杀虫剂的潜在靶点。有趣的是,几个先前确定的致病突变也位于同一接口中,这表明该接口对于通道门控非常重要。位于SPRY2-SPRY3接口中的另一个昆虫特异性回路可能会间接影响SPRY3和Repeat34之间的另一个门控接口。











































 京公网安备 11010802027423号
京公网安备 11010802027423号