Nature Microbiology ( IF 20.5 ) Pub Date : 2020-01-20 , DOI: 10.1038/s41564-019-0658-4 Rita A Oliveira 1 , Katharine M Ng 2 , Margarida B Correia 1 , Vitor Cabral 1 , Handuo Shi 2 , Justin L Sonnenburg 3, 4 , Kerwyn Casey Huang 2, 3, 4 , Karina B Xavier 1
|
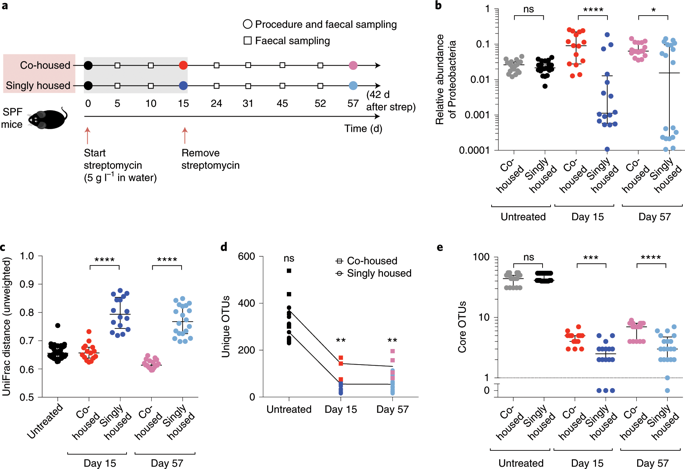
Intestinal microbiotas contain beneficial microorganisms that protect against pathogen colonization; treatment with antibiotics disrupts the microbiota and compromises colonization resistance. Here, we determine the impact of exchanging microorganisms between hosts on resilience to the colonization of invaders after antibiotic-induced dysbiosis. We assess the functional consequences of dysbiosis using a mouse model of colonization resistance against Escherichia coli. Antibiotics caused stochastic loss of members of the microbiota, but the microbiotas of co-housed mice remained more similar to each other compared with the microbiotas among singly housed animals. Strikingly, co-housed mice maintained colonization resistance after treatment with antibiotics, whereas most singly housed mice were susceptible to E. coli. The ability to retain or share the commensal Klebsiella michiganensis, a member of the Enterobacteriaceae family, was sufficient for colonization resistance after treatment with antibiotics. K. michiganensis generally outcompeted E. coli in vitro, but in vivo administration of galactitol—a nutrient that supports the growth of only E. coli—to bi-colonized gnotobiotic mice abolished the colonization-resistance capacity of K. michiganensis against E. coli, supporting the idea that nutrient competition is the primary interaction mechanism. K. michiganensis also hampered colonization of the pathogen Salmonella, prolonging host survival. Our results address functional consequences of the stochastic effects of microbiota perturbations, whereby microbial transmission through host interactions can facilitate reacquisition of beneficial commensals, minimizing the negative impact of antibiotics.
中文翻译:

密歇根克雷伯菌传播通过营养竞争增强对肠杆菌肠道入侵的抵抗力
肠道微生物群含有有益微生物,可防止病原体定植;抗生素治疗会破坏微生物群并损害定植抗性。在这里,我们确定了宿主之间交换微生物对抗生素引起的生态失调后入侵者定植的恢复能力的影响。我们使用针对大肠杆菌的定植抗性小鼠模型评估生态失调的功能后果。抗生素会导致微生物群成员的随机损失,但与单独饲养动物的微生物群相比,共同饲养小鼠的微生物群仍然更加相似。引人注目的是,共同饲养的小鼠在用抗生素治疗后保持定植抗性,而大多数单独饲养的小鼠对大肠杆菌。保留或共享肠杆菌科成员密歇根克雷伯菌的能力足以在抗生素治疗后产生定植抗性。K. michiganensis在体外通常胜过大肠杆菌,但在体内向双定殖的不育小鼠施用半乳糖醇(一种仅支持大肠杆菌生长的营养素)消除了K. michiganensis对大肠杆菌的定殖抗性能力, 支持养分竞争是主要相互作用机制的观点。K. michiganensis还阻碍病原体沙门氏菌的定植,延长宿主生存期。我们的结果解决了微生物群扰动的随机效应的功能后果,即微生物通过宿主相互作用传播可以促进有益共生体的重新获得,从而最大限度地减少抗生素的负面影响。









































 京公网安备 11010802027423号
京公网安备 11010802027423号