Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel invasion indices quantify the feed-forward facilitation of tumor invasion by macrophages.
Scientific Reports ( IF 3.8 ) Pub Date : 2020-01-20 , DOI: 10.1038/s41598-020-57517-6 Gippeum J Lim 1 , Suk-Jo Kang 1 , Ji Youn Lee 2
Scientific Reports ( IF 3.8 ) Pub Date : 2020-01-20 , DOI: 10.1038/s41598-020-57517-6 Gippeum J Lim 1 , Suk-Jo Kang 1 , Ji Youn Lee 2
Affiliation
|
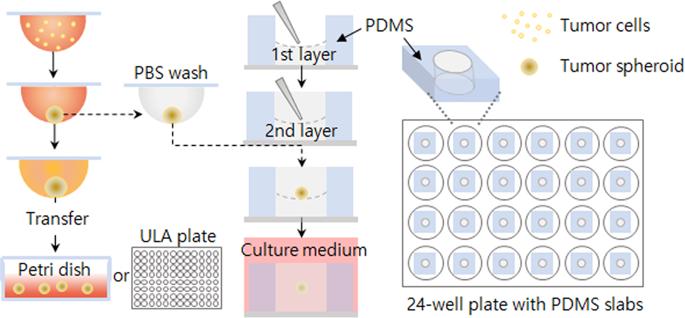
Quantitative and reliable measurement of cellular invasion is important to understand a range of biological processes such as cancer metastasis and angiogenesis. Spheroid invasion assays are an attractive in vitro platform because they effectively mimic the tumor cell invasion of solid tissues. Here, we developed an image analysis-based method to quantify the invasiveness of HT1080 human fibrosarcoma tumor cell spheroids. We segmented a cell-covered area into three subareas using objectively set threshold pixel intensities and calculated invasion indices using these subareas. Comparison with conventional parameters for spheroid invasion assays, such as area, length, and detached cells, showed that our indices present the invasion event at an early time and without being convoluted by proliferation. As an application, we then examined paracrine interactions between LLC1 mouse lung carcinoma cells and Raw264.7 mouse macrophage cells with our developed analysis method. We found that the invasion of tumor spheroids was increased by a macrophage-conditioned medium, concomitantly with a decrease in tumor cell proliferation. Importantly, invasion was further enhanced by a conditioned medium from activated macrophages by co-culture with tumor cells. Thus, our indices reveal that tumor cell invasion is facilitated in a feed-forward manner by communication between tumor cells and macrophages in the tumor microenvironment.
中文翻译:

新的侵袭指数量化了巨噬细胞对肿瘤侵袭的前馈促进作用。
定量和可靠地测量细胞浸润对于了解一系列生物学过程(例如癌症转移和血管生成)非常重要。球体入侵测定法是一种有吸引力的体外平台,因为它们有效地模仿了实体组织的肿瘤细胞侵袭。在这里,我们开发了一种基于图像分析的方法来量化HT1080人纤维肉瘤肿瘤细胞球体的侵袭性。我们使用客观设置的阈值像素强度将细胞覆盖的区域划分为三个子区域,并使用这些子区域计算入侵指数。与球体浸润测定的常规参数(例如面积,长度和分离的细胞)进行比较,结果表明,我们的指标能够在较早的时间内呈现浸润事件,而不会因增殖而复杂化。作为应用,然后,我们用我们开发的分析方法检查了LLC1小鼠肺癌细胞和Raw264.7小鼠巨噬细胞之间的旁分泌相互作用。我们发现巨噬细胞条件培养基增加了对肿瘤球体的侵袭,同时降低了肿瘤细胞的增殖。重要的是,通过与肿瘤细胞共培养,来自活化的巨噬细胞的条件培养基会进一步增强入侵。因此,我们的指标表明,肿瘤细胞与肿瘤微环境中的巨噬细胞之间的通讯以前馈方式促进了肿瘤细胞的侵袭。伴随肿瘤细胞增殖的减少。重要的是,通过与肿瘤细胞共培养,来自活化的巨噬细胞的条件培养基会进一步增强入侵。因此,我们的指标表明,肿瘤细胞与肿瘤微环境中的巨噬细胞之间的通讯以前馈方式促进了肿瘤细胞的侵袭。伴随肿瘤细胞增殖的减少。重要的是,通过与肿瘤细胞共培养,来自活化的巨噬细胞的条件培养基会进一步增强入侵。因此,我们的指标表明,肿瘤细胞与肿瘤微环境中的巨噬细胞之间的通讯以前馈方式促进了肿瘤细胞的侵袭。
更新日期:2020-01-21
中文翻译:

新的侵袭指数量化了巨噬细胞对肿瘤侵袭的前馈促进作用。
定量和可靠地测量细胞浸润对于了解一系列生物学过程(例如癌症转移和血管生成)非常重要。球体入侵测定法是一种有吸引力的体外平台,因为它们有效地模仿了实体组织的肿瘤细胞侵袭。在这里,我们开发了一种基于图像分析的方法来量化HT1080人纤维肉瘤肿瘤细胞球体的侵袭性。我们使用客观设置的阈值像素强度将细胞覆盖的区域划分为三个子区域,并使用这些子区域计算入侵指数。与球体浸润测定的常规参数(例如面积,长度和分离的细胞)进行比较,结果表明,我们的指标能够在较早的时间内呈现浸润事件,而不会因增殖而复杂化。作为应用,然后,我们用我们开发的分析方法检查了LLC1小鼠肺癌细胞和Raw264.7小鼠巨噬细胞之间的旁分泌相互作用。我们发现巨噬细胞条件培养基增加了对肿瘤球体的侵袭,同时降低了肿瘤细胞的增殖。重要的是,通过与肿瘤细胞共培养,来自活化的巨噬细胞的条件培养基会进一步增强入侵。因此,我们的指标表明,肿瘤细胞与肿瘤微环境中的巨噬细胞之间的通讯以前馈方式促进了肿瘤细胞的侵袭。伴随肿瘤细胞增殖的减少。重要的是,通过与肿瘤细胞共培养,来自活化的巨噬细胞的条件培养基会进一步增强入侵。因此,我们的指标表明,肿瘤细胞与肿瘤微环境中的巨噬细胞之间的通讯以前馈方式促进了肿瘤细胞的侵袭。伴随肿瘤细胞增殖的减少。重要的是,通过与肿瘤细胞共培养,来自活化的巨噬细胞的条件培养基会进一步增强入侵。因此,我们的指标表明,肿瘤细胞与肿瘤微环境中的巨噬细胞之间的通讯以前馈方式促进了肿瘤细胞的侵袭。











































 京公网安备 11010802027423号
京公网安备 11010802027423号