当前位置:
X-MOL 学术
›
Appl. Geochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Antimony mobility during the early stages of stibnite weathering in tailings at the Beaver Brook Sb deposit, Newfoundland
Applied Geochemistry ( IF 3.4 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.apgeochem.2020.104528 Anežka Borčinová Radková , Heather E. Jamieson , Kate M. Campbell
Applied Geochemistry ( IF 3.4 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.apgeochem.2020.104528 Anežka Borčinová Radková , Heather E. Jamieson , Kate M. Campbell
|
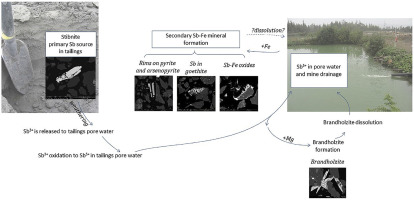
Abstract The aqueous speciation and mineralogy of antimony (Sb) in waters and tailings at Beaver Brook antimony deposit have been analyzed to understand Sb mobility during the initial stages of stibnite (Sb2S3) weathering in a near-surface environment. Dissolution of stibnite in oxidizing conditions releases Sb in drainage water and Sb is incorporated into the mineral structures of several secondary minerals. The most abundant Sb host in Beaver Brook tailings is primary stibnite, which dissolves, releasing Sb(III) to the pore water which rapidly oxidizes to Sb(V). The maximum concentration of Sb in tailings pore water is 26.4 mg/L and only 0.9% is in the form of Sb(III). In all surface water, Sb concentration ranges from 0.01 to 26.1 mg/L (average 9.4 mg/L) and is mostly present in its Sb(V) form (98.9–99.2% of total Sb). The secondary minerals containing Sb formed in tailings impoundment include tripuhyite-like Sb–Fe oxides (FeSbO4) where Sb is an important part of their structure, with variable Fe/Sb ratios and Sb concentrations of up to 37.8% by weight (average of 21.7%). These are important Sb host phases in the top 30 cm of tailings. Iron oxides enriched in Sb, such as goethite (FeOOH), where Sb (average of 3.9% by weight) is adsorbed or incorporated in the structure are common but represent less than 1.3% of the total mass of Sb. The elevated Mg concentrations in tailing ponds and pore water promote the precipitation of brandholzite (Mg[Sb(OH)6]2·6H2O) (in association with gypsum) during dry periods, which is easily dissolved during rainy periods. Brandholzite dissolution may significantly contribute to the concentration of dissolved Sb, together with stibnite dissolution, whereas Sb–Fe oxides are stable in the neutral pH, oxidized surface environment. Arsenic (As) accompanies Sb in all media but its behavior differs from that of Sb. The source of As is arsenopyrite, which decomposes more slowly than stibnite. This may be due to the formation of oxidation rims on arsenopyrite grains composed of Fe, As, S, Sb, and Ca which slow the dissolution, whereas no rims are seen on stibnite. Also, despite similar As and Sb concentration in bulk tailings, the concentration of Sb in drainage water is higher than that of As. In pore water, As(III) is the dominant oxidation state of As suggesting that the oxidation of dissolved As is slower than that of Sb.
中文翻译:

纽芬兰 Beaver Brook Sb 矿床尾矿中辉锑矿风化早期阶段的锑迁移率
摘要 分析了 Beaver Brook 锑矿床水和尾矿中锑 (Sb) 的含水形态和矿物学,以了解近地表环境中辉锑矿 (Sb2S3) 风化初期阶段的 Sb 迁移率。辉锑矿在氧化条件下的溶解会在废水中释放 Sb,并且 Sb 被结合到几种次生矿物的矿物结构中。Beaver Brook 尾矿中含量最丰富的 Sb 主体是初级辉锑矿,它溶解,将 Sb(III) 释放到孔隙水中,然后迅速氧化为 Sb(V)。尾矿孔隙水中 Sb 的最大浓度为 26.4 mg/L,只有 0.9% 以 Sb(III) 的形式存在。在所有地表水中,Sb 的浓度范围为 0.01 至 26.1 mg/L(平均 9.4 mg/L),并且主要以 Sb(V) 形式存在(占 Sb 总量的 98.9-99.2%)。在尾矿库中形成的含有 Sb 的次生矿物包括类似三重辉石的 Sb-Fe 氧化物 (FeSbO4),其中 Sb 是其结构的重要组成部分,具有可变的 Fe/Sb 比率和高达 37.8% 重量的 Sb 浓度(平均为 21.7 %)。这些是尾矿顶部 30 cm 中重要的 Sb 宿主相。富含 Sb 的氧化铁,如针铁矿 (FeOOH),其中 Sb(平均 3.9% 重量)被吸附或结合到结构中是常见的,但占 Sb 总质量的不到 1.3%。尾矿池和孔隙水中镁浓度的升高促进了白镁石(Mg[Sb(OH)6]2·6H2O)(与石膏结合)在干旱期的沉淀,在雨期容易溶解。Brandholzite 溶解可能对溶解 Sb 的浓度有显着影响,与辉锑矿溶解一起,而 Sb-Fe 氧化物在中性 pH 值、氧化的表面环境中是稳定的。砷 (As) 在所有介质中都伴随 Sb,但其行为与 Sb 不同。As 的来源是毒砂,其分解速度比辉锑矿慢。这可能是由于在由 Fe、As、S、Sb 和 Ca 组成的毒砂颗粒上形成了氧化边缘,这减缓了溶解,而在辉锑矿上没有看到氧化边缘。此外,尽管散装尾矿中的 As 和 Sb 浓度相似,但排水中的 Sb 浓度高于 As。在孔隙水中,As(III) 是 As 的主要氧化态,表明溶解的 As 的氧化比 Sb 的氧化慢。砷 (As) 在所有介质中都伴随 Sb,但其行为与 Sb 不同。As 的来源是毒砂,其分解速度比辉锑矿慢。这可能是由于在由 Fe、As、S、Sb 和 Ca 组成的毒砂颗粒上形成了氧化边缘,这减缓了溶解,而在辉锑矿上没有看到氧化边缘。此外,尽管散装尾矿中的 As 和 Sb 浓度相似,但排水中的 Sb 浓度高于 As。在孔隙水中,As(III) 是 As 的主要氧化态,表明溶解的 As 的氧化比 Sb 的氧化慢。砷 (As) 在所有介质中都伴随 Sb,但其行为与 Sb 不同。As 的来源是毒砂,其分解速度比辉锑矿慢。这可能是由于在由 Fe、As、S、Sb 和 Ca 组成的毒砂颗粒上形成了氧化边缘,这减缓了溶解,而在辉锑矿上没有看到氧化边缘。此外,尽管散装尾矿中的 As 和 Sb 浓度相似,但排水中的 Sb 浓度高于 As。在孔隙水中,As(III) 是 As 的主要氧化态,表明溶解的 As 的氧化比 Sb 的氧化慢。而辉锑矿上看不到边缘。此外,尽管散装尾矿中的 As 和 Sb 浓度相似,但排水中的 Sb 浓度高于 As。在孔隙水中,As(III) 是 As 的主要氧化态,表明溶解的 As 的氧化比 Sb 的氧化慢。而辉锑矿上看不到边缘。此外,尽管散装尾矿中的 As 和 Sb 浓度相似,但排水中的 Sb 浓度高于 As。在孔隙水中,As(III) 是 As 的主要氧化态,表明溶解的 As 的氧化比 Sb 的氧化慢。
更新日期:2020-04-01
中文翻译:

纽芬兰 Beaver Brook Sb 矿床尾矿中辉锑矿风化早期阶段的锑迁移率
摘要 分析了 Beaver Brook 锑矿床水和尾矿中锑 (Sb) 的含水形态和矿物学,以了解近地表环境中辉锑矿 (Sb2S3) 风化初期阶段的 Sb 迁移率。辉锑矿在氧化条件下的溶解会在废水中释放 Sb,并且 Sb 被结合到几种次生矿物的矿物结构中。Beaver Brook 尾矿中含量最丰富的 Sb 主体是初级辉锑矿,它溶解,将 Sb(III) 释放到孔隙水中,然后迅速氧化为 Sb(V)。尾矿孔隙水中 Sb 的最大浓度为 26.4 mg/L,只有 0.9% 以 Sb(III) 的形式存在。在所有地表水中,Sb 的浓度范围为 0.01 至 26.1 mg/L(平均 9.4 mg/L),并且主要以 Sb(V) 形式存在(占 Sb 总量的 98.9-99.2%)。在尾矿库中形成的含有 Sb 的次生矿物包括类似三重辉石的 Sb-Fe 氧化物 (FeSbO4),其中 Sb 是其结构的重要组成部分,具有可变的 Fe/Sb 比率和高达 37.8% 重量的 Sb 浓度(平均为 21.7 %)。这些是尾矿顶部 30 cm 中重要的 Sb 宿主相。富含 Sb 的氧化铁,如针铁矿 (FeOOH),其中 Sb(平均 3.9% 重量)被吸附或结合到结构中是常见的,但占 Sb 总质量的不到 1.3%。尾矿池和孔隙水中镁浓度的升高促进了白镁石(Mg[Sb(OH)6]2·6H2O)(与石膏结合)在干旱期的沉淀,在雨期容易溶解。Brandholzite 溶解可能对溶解 Sb 的浓度有显着影响,与辉锑矿溶解一起,而 Sb-Fe 氧化物在中性 pH 值、氧化的表面环境中是稳定的。砷 (As) 在所有介质中都伴随 Sb,但其行为与 Sb 不同。As 的来源是毒砂,其分解速度比辉锑矿慢。这可能是由于在由 Fe、As、S、Sb 和 Ca 组成的毒砂颗粒上形成了氧化边缘,这减缓了溶解,而在辉锑矿上没有看到氧化边缘。此外,尽管散装尾矿中的 As 和 Sb 浓度相似,但排水中的 Sb 浓度高于 As。在孔隙水中,As(III) 是 As 的主要氧化态,表明溶解的 As 的氧化比 Sb 的氧化慢。砷 (As) 在所有介质中都伴随 Sb,但其行为与 Sb 不同。As 的来源是毒砂,其分解速度比辉锑矿慢。这可能是由于在由 Fe、As、S、Sb 和 Ca 组成的毒砂颗粒上形成了氧化边缘,这减缓了溶解,而在辉锑矿上没有看到氧化边缘。此外,尽管散装尾矿中的 As 和 Sb 浓度相似,但排水中的 Sb 浓度高于 As。在孔隙水中,As(III) 是 As 的主要氧化态,表明溶解的 As 的氧化比 Sb 的氧化慢。砷 (As) 在所有介质中都伴随 Sb,但其行为与 Sb 不同。As 的来源是毒砂,其分解速度比辉锑矿慢。这可能是由于在由 Fe、As、S、Sb 和 Ca 组成的毒砂颗粒上形成了氧化边缘,这减缓了溶解,而在辉锑矿上没有看到氧化边缘。此外,尽管散装尾矿中的 As 和 Sb 浓度相似,但排水中的 Sb 浓度高于 As。在孔隙水中,As(III) 是 As 的主要氧化态,表明溶解的 As 的氧化比 Sb 的氧化慢。而辉锑矿上看不到边缘。此外,尽管散装尾矿中的 As 和 Sb 浓度相似,但排水中的 Sb 浓度高于 As。在孔隙水中,As(III) 是 As 的主要氧化态,表明溶解的 As 的氧化比 Sb 的氧化慢。而辉锑矿上看不到边缘。此外,尽管散装尾矿中的 As 和 Sb 浓度相似,但排水中的 Sb 浓度高于 As。在孔隙水中,As(III) 是 As 的主要氧化态,表明溶解的 As 的氧化比 Sb 的氧化慢。



























 京公网安备 11010802027423号
京公网安备 11010802027423号