当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of physicochemical properties of ammonium-based ionic liquids on CO2 capturing: An insight into structure-activity relationship
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.fluid.2020.112484 Sabahat Sardar , Asad Mumtaz , Mehwish Taneez , Masoom Yasinzai , Muhammad Imran Irshad , Jean-Marc Leveque
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.fluid.2020.112484 Sabahat Sardar , Asad Mumtaz , Mehwish Taneez , Masoom Yasinzai , Muhammad Imran Irshad , Jean-Marc Leveque
|
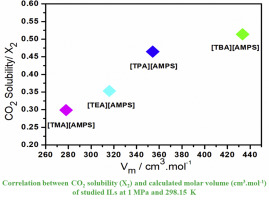
Abstract The physicochemical properties including density, viscosity and refractive index of five ionic liquids (ILs) consisting of 2-acrylamido-2-methylpropanesulfonate [AMPS] as anion source with ammonium-based cations (tetrabutylammonium [TBA], tetrapropylammonium [TPA] and tetraethylammonium [TEA], tetramethylammonium [TMA] and (vinylbenzyl)trimethylammonium [vbn]) were measured and influence of these properties upon CO2 sorption was investigated. The viscosity and density measurements were taken at atmospheric pressure within the temperature range of (293.15–363.15) K, whereas refractive indexes were determined within (288.15–333.15) K. An increase in the side-carbon chain length of ammonium-based cations resulted in decreased density with a corresponding increase in viscosity and refractive index. Among the studied ILs, [TBA][AMPS] gave the highest viscosity and least density over the whole range of temperature and a higher CO2 sorption of 0.51 mol fraction at 298.15 K and 1 MPa was observed showing dependency of CO2 solubility on the thermophysical properties of studied ILs. Moreover, among investigated ILs, molar volume of [TBA][AMPS] was found to be largest (433.3729 cm3mol-1), which resulted in the higher amount of CO2 sorption in comparison to other investigated ILs. Also, Henry's constant of 1.51 MPa was found for [TBA][AMPS] which was 10.9, 33.4, 44.3 and 24.8% less than [TPA][AMPS], [TEA][AMPS], [TMA][AMPS] and [vbn][AMPS], respectively. The current work offers an in depth study to understand structure-activity relation between physicochemical properties and CO2 solubility of investigated ILs and explores the ways for enhanced CO2 sorption.
中文翻译:

铵基离子液体理化性质对 CO2 捕获的影响:构效关系的洞察
摘要 以 2-丙烯酰胺基-2-甲基丙磺酸盐 [AMPS] 作为阴离子源和铵基阳离子(四丁基铵 [TBA]、四丙基铵 [TPA] 和四乙基铵)组成的五种离子液体 (ILs) 的密度、粘度和折射率等物理化学性质测量了 [TEA]、四甲基铵 [TMA] 和(乙烯基苄基)三甲基铵 [vbn]),并研究了这些特性对 CO2 吸附的影响。粘度和密度测量是在 (293.15-363.15) K 温度范围内的大气压下进行的,而折射率在 (288.15-333.15) K 内确定。导致铵基阳离子的侧碳链长度增加密度降低,粘度和折射率相应增加。在研究的 ILs 中,[TBA][AMPS] 在整个温度范围内具有最高的粘度和最低的密度,并且在 298.15 K 和 1 MPa 下观察到 0.51 摩尔分数的较高 CO2 吸附,表明 CO2 溶解度对所研究的离子液体的热物理性质有依赖性。此外,在研究的 ILs 中,发现 [TBA][AMPS] 的摩尔体积最大 (433.3729 cm3mol-1),与其他研究的 ILs 相比,这导致更高的 CO2 吸附量。此外,[TBA][AMPS] 的亨利常数为 1.51 MPa,比 [TPA][AMPS]、[TEA][AMPS]、[TMA][AMPS] 和 [AMPS] 小 10.9、33.4、44.3 和 24.8% vbn][AMPS],分别。目前的工作提供了深入研究,以了解所研究 IL 的理化性质与 CO2 溶解度之间的构效关系,并探索增强 CO2 吸附的方法。
更新日期:2020-04-01
中文翻译:

铵基离子液体理化性质对 CO2 捕获的影响:构效关系的洞察
摘要 以 2-丙烯酰胺基-2-甲基丙磺酸盐 [AMPS] 作为阴离子源和铵基阳离子(四丁基铵 [TBA]、四丙基铵 [TPA] 和四乙基铵)组成的五种离子液体 (ILs) 的密度、粘度和折射率等物理化学性质测量了 [TEA]、四甲基铵 [TMA] 和(乙烯基苄基)三甲基铵 [vbn]),并研究了这些特性对 CO2 吸附的影响。粘度和密度测量是在 (293.15-363.15) K 温度范围内的大气压下进行的,而折射率在 (288.15-333.15) K 内确定。导致铵基阳离子的侧碳链长度增加密度降低,粘度和折射率相应增加。在研究的 ILs 中,[TBA][AMPS] 在整个温度范围内具有最高的粘度和最低的密度,并且在 298.15 K 和 1 MPa 下观察到 0.51 摩尔分数的较高 CO2 吸附,表明 CO2 溶解度对所研究的离子液体的热物理性质有依赖性。此外,在研究的 ILs 中,发现 [TBA][AMPS] 的摩尔体积最大 (433.3729 cm3mol-1),与其他研究的 ILs 相比,这导致更高的 CO2 吸附量。此外,[TBA][AMPS] 的亨利常数为 1.51 MPa,比 [TPA][AMPS]、[TEA][AMPS]、[TMA][AMPS] 和 [AMPS] 小 10.9、33.4、44.3 和 24.8% vbn][AMPS],分别。目前的工作提供了深入研究,以了解所研究 IL 的理化性质与 CO2 溶解度之间的构效关系,并探索增强 CO2 吸附的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号