当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and biological activity evaluation of a series of pleuromutilin derivatives with novel C14 side chains.
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-01-18 , DOI: 10.1016/j.bmcl.2020.126969 Yun-Ge Li 1 , Ju-Xian Wang 2 , Guo-Ning Zhang 2 , Mei Zhu 2 , Xue-Fu You 2 , Yu-Cheng Wang 2 , Fan Zhang 1
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-01-18 , DOI: 10.1016/j.bmcl.2020.126969 Yun-Ge Li 1 , Ju-Xian Wang 2 , Guo-Ning Zhang 2 , Mei Zhu 2 , Xue-Fu You 2 , Yu-Cheng Wang 2 , Fan Zhang 1
Affiliation
|
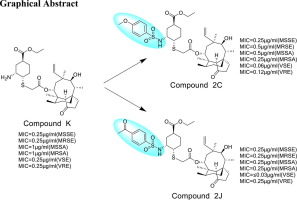
In this work, according to the 'me-too me-better' design strategy, a peculiar side chain different from lefamulin at C14 position of pleuromutilin was introduced. A series of novel thioether pleuromutilin derivatives containing cyclohexane in the C14 chain was synthesized by ten-step synthesis reaction. All derivatives were characterized by Nuclear Magnetic Resonance (NMR) and High Resolution Mass Spectrometer (HRMS). Furthermore, majority of derivatives displayed moderate antibacterial activity in vitro. However, the compound 2C and 2J exhibited comparable or superior antibacterial activity to lefamulin. The summarized structure-activity relationship not only made the variety of pleuromutilin derivatives more diverse, but also provided new ideas for its design and development.
中文翻译:

一系列具有新型C14侧链的截短侧耳素衍生物的设计、合成及生物活性评价。
本工作根据“me-too me-better”的设计策略,在截短侧耳素的C14位上引入了一条不同于lefamulin的奇特侧链。通过十步合成反应合成了一系列C14链上含有环己烷的新型硫醚截短侧耳素衍生物。所有衍生物均通过核磁共振 (NMR) 和高分辨率质谱仪 (HRMS) 进行表征。此外,大多数衍生物在体外表现出中等的抗菌活性。然而,化合物2C和2J表现出与来法莫林相当或更好的抗菌活性。总结的构效关系不仅使截短侧耳素衍生物的品种更加丰富,而且为其设计和开发提供了新的思路。
更新日期:2020-01-21
中文翻译:

一系列具有新型C14侧链的截短侧耳素衍生物的设计、合成及生物活性评价。
本工作根据“me-too me-better”的设计策略,在截短侧耳素的C14位上引入了一条不同于lefamulin的奇特侧链。通过十步合成反应合成了一系列C14链上含有环己烷的新型硫醚截短侧耳素衍生物。所有衍生物均通过核磁共振 (NMR) 和高分辨率质谱仪 (HRMS) 进行表征。此外,大多数衍生物在体外表现出中等的抗菌活性。然而,化合物2C和2J表现出与来法莫林相当或更好的抗菌活性。总结的构效关系不仅使截短侧耳素衍生物的品种更加丰富,而且为其设计和开发提供了新的思路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号