Tetrahedron ( IF 2.1 ) Pub Date : 2020-01-18 , DOI: 10.1016/j.tet.2020.130965 Tamás Nemcsok , Zsolt Rapi , Péter Bagi , Ying Hou Guan , István Orbán , György Keglevich , Péter Bakó
|
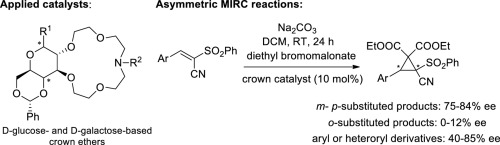
A few new d-galactose- and d-glucose-based monoaza-15-crown-5 type lariat ethers have been synthesized. These macrocycles and their derivatives proved to be efficient catalysts in the cyclopropanation of (E)-3-phenyl-2-(phenylsulfonyl)acrylonitrile performed with diethyl bromomalonate under mild phase transfer conditions. Among the catalysts tested, the macrocycle having methyl α-d-galactopyranoside unit generated the highest asymmetric induction (80% ee). In the reactions of the aryl-substituted phenylsulfonyl-acrylonitrile derivatives, the cyclopropanation of the meta- and para-substituted starting materials took place with high ee values (75–84% ee). The cyclopropane derivatives synthesized from analogous α,β-unsaturated cyanosulfones containing naphthyl, pyridyl, furyl and thienyl groups were obtained with enantioselectivities up to 85%, and in excellent yields.
中文翻译:

使用基于碳水化合物的冠醚催化剂对共轭氰砜的对映选择性环丙烷化
已经合成了一些新的基于d-半乳糖和d-葡萄糖的monoaza-15-crown-5型套索状醚。这些大环及其衍生物被证明是在温和的相转移条件下用溴代丙二酸二乙酯对(E)-3-苯基-2-(苯磺酰基)丙烯腈进行环丙烷化反应的有效催化剂。在所测试的催化剂中,具有甲基α- d-吡喃半乳糖苷单元的大环化合物产生最高的不对称诱导(80%ee)。在芳基取代的苯磺酰基-丙烯腈衍生物的反应中,间位和对位的环丙烷化发生了高ee值(75-84%ee)的替代原料。由具有萘基,吡啶基,呋喃基和噻吩基的类似的α,β-不饱和氰砜合成的环丙烷衍生物的对映选择性高达85%,收率很高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号