当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unexpected migration of a benzoyl group in the intramolecular Wittig reaction of o-acyloxybenzylidenephosphoranes with benzoyl chlorides: one-pot synthesis of isomeric 3-benzoyl-2-phenylbenzofurans
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-01-20 , DOI: 10.1016/j.tetlet.2020.151634 Michela Begala , Michele Mancinelli , Giovanna Lucia Delogu
中文翻译:

邻酰氧基亚苄基膦酸酯与苯甲酰氯的分子内Wittig反应中苯甲酰基的意外迁移:异构体3-苯甲酰基-2-苯基苯并呋喃的一锅合成
更新日期:2020-01-21
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-01-20 , DOI: 10.1016/j.tetlet.2020.151634 Michela Begala , Michele Mancinelli , Giovanna Lucia Delogu
|
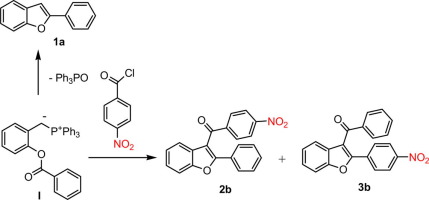
The reaction of o-acyloxybenzylidenetriphenylphosphoranes with substituted benzoyl chlorides in an aprotic solvent led, together with the expected 2-phenylbenzofuran, to isomeric 3-benzoyl-2-phenylbenzofuran derivatives. This result formally corresponds to intramolecular migration of the benzoyl group from the ortho oxygen atom to the ylide carbanion via cyclization and ring opening of the starting o-acyloxybenzylidenetriphenylphosphoranes.
中文翻译:

邻酰氧基亚苄基膦酸酯与苯甲酰氯的分子内Wittig反应中苯甲酰基的意外迁移:异构体3-苯甲酰基-2-苯基苯并呋喃的一锅合成
在非质子溶剂中,邻-酰氧基苄基亚氨基三苯基苯基膦与取代的苯甲酰氯的反应与预期的2-苯基苯并呋喃一起导致异构体3-苯甲酰基-2-苯基苯并呋喃衍生物。该结果形式上对应于苯甲酰基从邻氧原子通过环化和起始的邻-酰氧基苄基亚氨基三苯基磷ora烷的开环从分子内迁移至内酰碳负离子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号