当前位置:
X-MOL 学术
›
Cell Death Differ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dissecting DISC regulation via pharmacological targeting of caspase-8/c-FLIPL heterodimer.
Cell Death and Differentiation ( IF 13.7 ) Pub Date : 2020-01-20 , DOI: 10.1038/s41418-020-0489-0 Laura K Hillert 1 , Nikita V Ivanisenko 2 , Denise Busse 1 , Johannes Espe 1 , Corinna König 1 , Sergey E Peltek 2 , Nikolai A Kolchanov 2 , Vladimir A Ivanisenko 2 , Inna N Lavrik 1
Cell Death and Differentiation ( IF 13.7 ) Pub Date : 2020-01-20 , DOI: 10.1038/s41418-020-0489-0 Laura K Hillert 1 , Nikita V Ivanisenko 2 , Denise Busse 1 , Johannes Espe 1 , Corinna König 1 , Sergey E Peltek 2 , Nikolai A Kolchanov 2 , Vladimir A Ivanisenko 2 , Inna N Lavrik 1
Affiliation
|
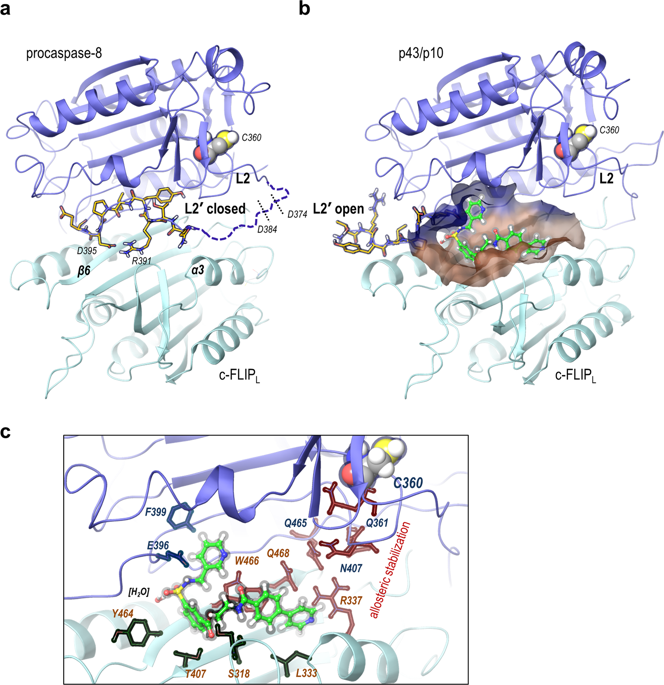
Pharmacological targeting via small molecule-based chemical probes has recently acquired an emerging importance as a valuable tool to delineate molecular mechanisms. Induction of apoptosis via CD95/Fas and TRAIL-R1/2 is triggered by the formation of the death-inducing signaling complex (DISC). Caspase-8 activation at the DISC is largely controlled by c-FLIP proteins. However molecular mechanisms of this control have just started to be uncovered. In this study we report the first-in-class chemical probe targeting c-FLIPL in the heterodimer caspase-8/c-FLIPL. This rationally designed small molecule was aimed to imitate the closed conformation of the caspase-8 L2' loop and thereby increase caspase-8 activity after initial processing of the heterodimer. In accordance with in silico predictions, this small molecule enhanced caspase-8 activity at the DISC, CD95L/TRAIL-induced caspase activation, and subsequent apoptosis. The generated computational model provided further evidence for the proposed effects of the small molecule on the heterodimer caspase-8/c-FLIPL. In particular, the model has demonstrated that boosting caspase-8 activity by the small molecule at the early time points after DISC assembly is crucial for promoting apoptosis induction. Taken together, our study allowed to target the heterodimer caspase-8/c-FLIPL and get new insights into molecular mechanisms of its activation.
中文翻译:

通过 caspase-8/c-FLIPL 异二聚体的药理学靶向剖析 DISC 调控。
通过基于小分子的化学探针的药理学靶向最近已成为描述分子机制的重要工具。通过 CD95/Fas 和 TRAIL-R1/2 诱导细胞凋亡是由死亡诱导信号复合物 (DISC) 的形成触发的。DISC 上的 Caspase-8 激活主要由 c-FLIP 蛋白控制。然而,这种控制的分子机制才刚刚开始被发现。在这项研究中,我们报告了在异二聚体 caspase-8/c-FLIPL 中靶向 c-FLIPL 的一流化学探针。这种设计合理的小分子旨在模仿 caspase-8 L2' 环的闭合构象,从而在异源二聚体初始加工后增加 caspase-8 的活性。根据硅片预测,这种小分子增强了 DISC 中的 caspase-8 活性、CD95L/TRAIL 诱导的 caspase 激活和随后的细胞凋亡。生成的计算模型为小分子对异二聚体 caspase-8/c-FLIPL 的拟议影响提供了进一步的证据。特别是,该模型已经证明,小分子在 DISC 组装后的早期时间点提高 caspase-8 活性对于促进细胞凋亡诱导至关重要。总之,我们的研究允许靶向异二聚体 caspase-8/c-FLIPL 并对其激活的分子机制有新的了解。该模型表明,在 DISC 组装后的早期时间点通过小分子提高 caspase-8 活性对于促进细胞凋亡诱导至关重要。总之,我们的研究允许靶向异二聚体 caspase-8/c-FLIPL 并对其激活的分子机制有新的了解。该模型表明,在 DISC 组装后的早期时间点通过小分子提高 caspase-8 活性对于促进细胞凋亡诱导至关重要。总之,我们的研究允许靶向异二聚体 caspase-8/c-FLIPL 并对其激活的分子机制有新的了解。
更新日期:2020-01-20
中文翻译:

通过 caspase-8/c-FLIPL 异二聚体的药理学靶向剖析 DISC 调控。
通过基于小分子的化学探针的药理学靶向最近已成为描述分子机制的重要工具。通过 CD95/Fas 和 TRAIL-R1/2 诱导细胞凋亡是由死亡诱导信号复合物 (DISC) 的形成触发的。DISC 上的 Caspase-8 激活主要由 c-FLIP 蛋白控制。然而,这种控制的分子机制才刚刚开始被发现。在这项研究中,我们报告了在异二聚体 caspase-8/c-FLIPL 中靶向 c-FLIPL 的一流化学探针。这种设计合理的小分子旨在模仿 caspase-8 L2' 环的闭合构象,从而在异源二聚体初始加工后增加 caspase-8 的活性。根据硅片预测,这种小分子增强了 DISC 中的 caspase-8 活性、CD95L/TRAIL 诱导的 caspase 激活和随后的细胞凋亡。生成的计算模型为小分子对异二聚体 caspase-8/c-FLIPL 的拟议影响提供了进一步的证据。特别是,该模型已经证明,小分子在 DISC 组装后的早期时间点提高 caspase-8 活性对于促进细胞凋亡诱导至关重要。总之,我们的研究允许靶向异二聚体 caspase-8/c-FLIPL 并对其激活的分子机制有新的了解。该模型表明,在 DISC 组装后的早期时间点通过小分子提高 caspase-8 活性对于促进细胞凋亡诱导至关重要。总之,我们的研究允许靶向异二聚体 caspase-8/c-FLIPL 并对其激活的分子机制有新的了解。该模型表明,在 DISC 组装后的早期时间点通过小分子提高 caspase-8 活性对于促进细胞凋亡诱导至关重要。总之,我们的研究允许靶向异二聚体 caspase-8/c-FLIPL 并对其激活的分子机制有新的了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号