当前位置:
X-MOL 学术
›
Colloids Surf. B Biointerfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Camel α-lactalbumin at the oil-water interface: Effect of protein concentration and pH change on surface characteristics and emulsifying properties.
Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2020-01-20 , DOI: 10.1016/j.colsurfb.2019.110654 Maroua Ellouze 1 , Roua Lajnaf 2 , Ahmed Zouari 2 , Hamadi Attia 2 , Mohamed Ali Ayadi 2 , Christophe Vial 3
Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2020-01-20 , DOI: 10.1016/j.colsurfb.2019.110654 Maroua Ellouze 1 , Roua Lajnaf 2 , Ahmed Zouari 2 , Hamadi Attia 2 , Mohamed Ali Ayadi 2 , Christophe Vial 3
Affiliation
|
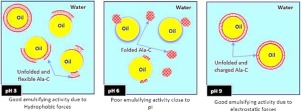
Camel α-lactalbumin (Ala-C), the main whey protein of camel milk, was purified by membrane filtration. Surface hydrophobicity as well as interfacial tension were examined at different levels of pH (3.0, 6.0, 9.0) and protein concentration (0.1 %, 0.2 %, 0.4 % w/w), and compared to bovine α-lactalbumin (Ala-B). The emulsifying properties (EAI and ESI) of oil-in-water emulsions (20 %/80 %) were investigated for both proteins. The stability of the processed emulsions was characterised by ζ-potential, particle size and viscosity measurements. The main findings indicate that Ala-C exhibited greater surface hydrophobicity and undergone changes in conformational structure when pH decreased from 9.0-3.0. These changes were enhanced by increasing protein concentration from 0.1 % to 0.4 % (w/w). However, high concentrations showed low emulsifying activity, especially at pH 6.0 where interfacial tension was lower. In comparison with Ala-B, maximum EAI was close, despite the lower surface hydrophobicity of Ala-C under similar conditions. Overall, emulsions were more viscous at pH 3.0 due to the greater surface coverage than at 9.0 and 6.0. Under the conditions of this study, a protein concentration of 0.2 % resulted in the finest oil droplets and highest viscosity for both types of α-lactalbumin, and Ala-C conferred the highest long-term stability to the emulsions.
中文翻译:

油水界面上的骆驼α-乳白蛋白:蛋白质浓度和pH值变化对表面特性和乳化特性的影响。
骆驼奶的主要乳清蛋白骆驼α-乳白蛋白(Ala-C)通过膜过滤纯化。在不同的pH值(3.0、6.0、9.0)和蛋白质浓度(0.1%,0.2%,0.4%w / w)下检查表面疏水性和界面张力,并与牛α-乳白蛋白(Ala-B)进行比较。研究了两种蛋白的水包油乳液(20%/ 80%)的乳化性能(EAI和ESI)。通过ζ电势,粒度和粘度测量来表征加工的乳液的稳定性。主要发现表明,当pH从9.0-3.0降低时,Ala-C表现出更大的表面疏水性并发生构象结构变化。通过将蛋白质浓度从0.1%增加到0.4%(w / w),可以增强这些变化。但是,高浓度时乳化活性低,特别是在pH 6.0时,界面张力较低。与Ala-B相比,尽管在类似条件下Ala-C的表面疏水性较低,但最大EAI却很接近。总体而言,由于pH值大于9.0和6.0,乳化剂的表面覆盖率更高,因此在pH 3.0时的粘性更大。在这项研究的条件下,蛋白质浓度为0.2%时,两种类型的α-乳清蛋白均具有最细的油滴和最高的粘度,而Ala-C赋予乳液最高的长期稳定性。
更新日期:2020-01-21
中文翻译:

油水界面上的骆驼α-乳白蛋白:蛋白质浓度和pH值变化对表面特性和乳化特性的影响。
骆驼奶的主要乳清蛋白骆驼α-乳白蛋白(Ala-C)通过膜过滤纯化。在不同的pH值(3.0、6.0、9.0)和蛋白质浓度(0.1%,0.2%,0.4%w / w)下检查表面疏水性和界面张力,并与牛α-乳白蛋白(Ala-B)进行比较。研究了两种蛋白的水包油乳液(20%/ 80%)的乳化性能(EAI和ESI)。通过ζ电势,粒度和粘度测量来表征加工的乳液的稳定性。主要发现表明,当pH从9.0-3.0降低时,Ala-C表现出更大的表面疏水性并发生构象结构变化。通过将蛋白质浓度从0.1%增加到0.4%(w / w),可以增强这些变化。但是,高浓度时乳化活性低,特别是在pH 6.0时,界面张力较低。与Ala-B相比,尽管在类似条件下Ala-C的表面疏水性较低,但最大EAI却很接近。总体而言,由于pH值大于9.0和6.0,乳化剂的表面覆盖率更高,因此在pH 3.0时的粘性更大。在这项研究的条件下,蛋白质浓度为0.2%时,两种类型的α-乳清蛋白均具有最细的油滴和最高的粘度,而Ala-C赋予乳液最高的长期稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号