当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional Tolerance to Cysteine Mutations in Human α7 Nicotinic Acetylcholine Receptors.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-01-22 , DOI: 10.1021/acschemneuro.9b00647 Tommy S Tillman 1 , Zachary Choi 1 , Yan Xu 1, 2, 3, 4 , Pei Tang 1, 2, 5
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-01-22 , DOI: 10.1021/acschemneuro.9b00647 Tommy S Tillman 1 , Zachary Choi 1 , Yan Xu 1, 2, 3, 4 , Pei Tang 1, 2, 5
Affiliation
|
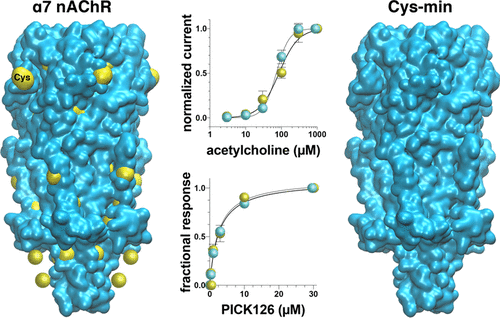
The α7 nicotinic acetylcholine receptor (α7 nAChR) is involved in various intracellular signaling pathways that mediate addiction, chronic pain, and other diseases, but its intracellular domain structures remain undetermined. The presence of 17 native cysteines in α7 nAChR provides opportunities for extracting structural information through site-directed labeling of chemical probes in strategic locations, but it also creates uncertainties in channel function when those native cysteines must be mutated. Using site-directed mutagenesis and two-electrode voltage clamp electrophysiology measurements, we found that α7 nAChR's function was well tolerated for mutations of all 13 cysteines as long as two pairs of disulfide-bond cysteines remained in the extracellular domain. Furthermore, surface plasmon resonance measurements showed that the cysteine mutations did not affect α7 nAChR binding to the intracellular protein PICK1. The study suggests that a high native cysteine content does not necessarily preclude the use of single cysteine labeling for acquiring structural information on functional proteins.
中文翻译:

对人α7烟碱乙酰胆碱受体半胱氨酸突变的功能耐受性。
α7烟碱乙酰胆碱受体(α7nAChR)参与介导成瘾,慢性疼痛和其他疾病的各种细胞内信号传导途径,但其细胞内结构域结构尚未确定。α7nAChR中17个天然半胱氨酸的存在为通过在战略位置化学探针进行定点标记提供了提取结构信息的机会,但是当这些天然半胱氨酸必须突变时,也为通道功能带来了不确定性。使用定点诱变和两电极电压钳电生理测量,我们发现只要两对二硫键半胱氨酸保留在细胞外结构域,α7nAChR的功能就可以耐受所有13个半胱氨酸的突变。此外,表面等离子体共振测量表明,半胱氨酸突变不影响α7nAChR与细胞内蛋白PICK1的结合。该研究表明,高天然半胱氨酸含量并不一定排除使用单个半胱氨酸标记获得功能蛋白的结构信息。
更新日期:2020-01-23
中文翻译:

对人α7烟碱乙酰胆碱受体半胱氨酸突变的功能耐受性。
α7烟碱乙酰胆碱受体(α7nAChR)参与介导成瘾,慢性疼痛和其他疾病的各种细胞内信号传导途径,但其细胞内结构域结构尚未确定。α7nAChR中17个天然半胱氨酸的存在为通过在战略位置化学探针进行定点标记提供了提取结构信息的机会,但是当这些天然半胱氨酸必须突变时,也为通道功能带来了不确定性。使用定点诱变和两电极电压钳电生理测量,我们发现只要两对二硫键半胱氨酸保留在细胞外结构域,α7nAChR的功能就可以耐受所有13个半胱氨酸的突变。此外,表面等离子体共振测量表明,半胱氨酸突变不影响α7nAChR与细胞内蛋白PICK1的结合。该研究表明,高天然半胱氨酸含量并不一定排除使用单个半胱氨酸标记获得功能蛋白的结构信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号