Theranostics ( IF 12.4 ) Pub Date : 2020-01-01 , DOI: 10.7150/thno.41708 Jun Dai 1 , Min Xu 2 , Quan Wang 2 , Juliang Yang 2 , Jinjin Zhang 1 , Pengfei Cui 1 , Wenwen Wang 1 , Xiaoding Lou 2 , Fan Xia 2 , Shixuan Wang 1
|
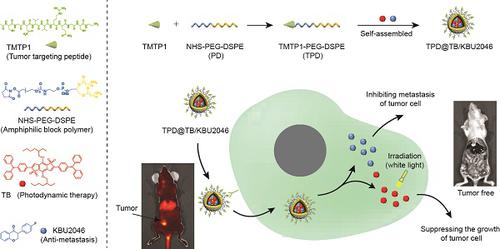
Metastasis is one of the main causes of death and treatment failure in ovarian cancer. Some small molecule inhibitors can effectively inhibit the metastasis of primary tumors. However, they do not kill the primary tumor cells, which may lead to continuous proliferation. Herein, we have prepared a multifunctional nanoparticles named TPD@TB/KBU2046, which consisted of three functional moieties: (1) KBU2046 (small molecule inhibitor) that can inhibit the metastasis of the primary tumors, (2) TB (photodynamic-AIEgens) that may suppress the growth of the primary tumors, and (3) TPD, which contains TMTP1 (a targeting peptide, which specifically binds to highly metastatic tumor cells) that can enhance the TB/KBU2046 dosage in the tumor site.
Methods: The TPD@TB/KBU2046 was prepared by nano-precipitation method. We linked the targeting peptide (TMTP1) to the nanoparticles via amidation reaction. TPD@TB/KBU2046 nanoparticles were characterized for encapsulation efficiency, particle size, absorption spectra, emission spectra and ROS production. The combinational efficacy in image-guided anti-metastasis and photodynamic therapy of TPD@TB/KBU2046 was explored both in vitro and in vivo.
Results: The TPD@TB/KBU2046 showed an average hydrodynamic size of approximately 50 nm with good stability. In vitro, TPD@TB/KBU2046 not only inhibited the metastasis of the tumors, but also suppressed the growth of the tumors under AIEgens-mediated photodynamic therapy. In vivo, we confirmed that TPD@TB/KBU2046 has the therapeutic effects of anti-tumor growth and anti-metastasis through subcutaneous and orthotopic ovarian tumor models.
Conclusion: Our findings provided an effective strategy to compensate for the congenital defects of some small molecule inhibitors and thus enhanced the therapeutic efficacy of ovarian cancer.
中文翻译:

卵巢癌中光动力AIEgens抗生长与小分子抑制剂抗转移之间的协同治疗。
转移是卵巢癌死亡和治疗失败的主要原因之一。一些小分子抑制剂可以有效抑制原发性肿瘤的转移。但是,它们不会杀死原发性肿瘤细胞,这可能导致持续增殖。本文中,我们制备了一种多功能的纳米颗粒TPD @ TB / KBU2046,它由三个功能部分组成:(1)可以抑制原发肿瘤转移的KBU2046(小分子抑制剂),(2)TB(光动力AIEgens) (3)TPD,它含有可增强肿瘤部位TB / KBU2046剂量的TMTP1(一种特异性结合高度转移性肿瘤细胞的靶向肽),可抑制肿瘤的生长。
方法:采用纳米沉淀法制备TPD @ TB / KBU2046。我们通过酰胺化反应将靶向肽(TMTP1)连接到纳米颗粒。TPD @ TB / KBU2046纳米粒子的包封效率,粒径,吸收光谱,发射光谱和活性氧产生的特点。在图像引导的抗转移和TPD @ TB / KBU2046的光动力治疗的组合功效都进行了探讨在体外和体内。
结果: TPD @ TB / KBU2046显示出约50 nm的平均流体动力学尺寸,并具有良好的稳定性。在体外,TPD @ TB / KBU2046不仅在AIEgens介导的光动力疗法的作用下抑制了肿瘤的转移,而且抑制了肿瘤的生长。在体内,我们证实TPD @ TB / KBU2046通过皮下和原位卵巢肿瘤模型具有抗肿瘤生长和抗转移的治疗作用。
结论:我们的发现为补偿某些小分子抑制剂的先天性缺陷提供了有效的策略,从而提高了卵巢癌的治疗效果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号