Theranostics ( IF 12.4 ) Pub Date : 2020-01-01 , DOI: 10.7150/thno.40395 Hui Yu 1 , Feiyang Jin 1 , Di Liu 1 , Gaofeng Shu 1 , Xiaojuan Wang 2 , Jing Qi 1 , Mingchen Sun 1 , Ping Yang 2 , Saiping Jiang 2 , Xiaoying Ying 1 , Yongzhong Du 1
|
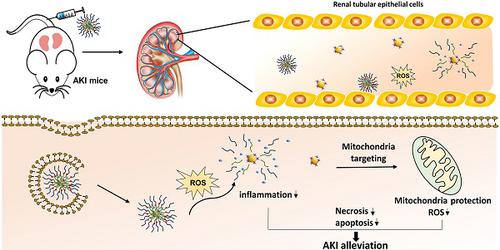
Acute kidney injury (AKI) caused by sepsis is a serious disease which mitochondrial oxidative stress and inflammatory play a key role in its pathophysiology. Ceria nanoparticles hold strong and recyclable reactive oxygen species (ROS)-scavenging activity, have been applied to treat ROS-related diseases. However, ceria nanoparticles can't selectively target mitochondria and the ultra-small ceria nanoparticles are easily agglomerated. To overcome these shortcomings and improve therapeutic efficiency, we designed an ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury.
Methods: Ceria nanoparticles were modified with triphenylphosphine (TCeria NPs), followed by coating with ROS-responsive organic polymer (mPEG-TK-PLGA) and loaded atorvastatin (Atv/PTP-TCeria NPs). The physicochemical properties, in vitro drug release profiles, mitochondria-targeting ability, in vitro antioxidant, anti-apoptotic activity and in vivo treatment efficacy of Atv/PTP-TCeria NPs were examined.
Results: Atv/PTP-TCeria NPs could accumulate in kidneys and hold a great ability to ROS-responsively release drug and TCeria NPs could target mitochondria to eliminate excessive ROS. In vitro study suggested Atv/PTP-TCeria NPs exhibited superior antioxidant and anti-apoptotic activity. In vivo study showed that Atv/PTP-TCeria NPs effectively decreased oxidative stress and inflammatory, could protect the mitochondrial structure, reduced apoptosis of tubular cell and tubular necrosis in the sepsis-induced AKI mice model.
Conclusions: This ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin has favorable potentials in the sepsis-induced AKI therapy.
中文翻译:

ROS响应纳米药物输送系统,结合针对线粒体的氧化铈纳米颗粒与阿托伐他汀,用于急性肾损伤。
败血症引起的急性肾损伤(AKI)是一种严重的疾病,线粒体的氧化应激和炎症在其病理生理中起关键作用。二氧化铈纳米粒子具有很强的可回收活性氧清除活性,已被用于治疗与ROS有关的疾病。但是,二氧化铈纳米粒子不能选择性地靶向线粒体,超小二氧化铈纳米粒子容易团聚。为了克服这些缺点并提高治疗效率,我们设计了一种针对ROS的纳米药物递送系统,该系统将靶向线粒体的氧化铈纳米粒子与阿托伐他汀相结合,用于急性肾脏损伤。
方法:用三苯膦(TCeria NPs)修饰二氧化铈纳米颗粒,然后用ROS反应性有机聚合物(mPEG-TK-PLGA)和负载的阿托伐他汀(Atv / PTP-TCeria NPs)涂覆。检查了Atv / PTP-TCeria NP的理化特性,体外药物释放曲线,线粒体靶向能力,体外抗氧化剂,抗凋亡活性和体内治疗功效。
结果:Atv / PTP-TCeria NPs可以在肾脏中积累,并具有较强的ROS响应释放药物的能力,TCeria NPs可以靶向线粒体以消除过量的ROS。体外研究表明Atv / PTP-TCeria NP表现出优异的抗氧化和抗凋亡活性。体内研究表明,在脓毒症诱发的AKI小鼠模型中,Atv / PTP-TCeria NPs可以有效降低氧化应激和炎症反应,可以保护线粒体结构,减少肾小管细胞凋亡和肾小管坏死。
结论:结合线粒体靶向二氧化铈纳米粒子与阿托伐他汀的这种ROS响应纳米药物递送系统在脓毒症诱发的AKI治疗中具有良好的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号