Theranostics ( IF 12.4 ) Pub Date : 2020-01-01 , DOI: 10.7150/thno.39238 Yin Hu 1, 2 , Yan Zhang 1, 3, 4 , Chu-Yu Ni 2 , Chun-Yuan Chen 1, 3 , Shan-Shan Rao 3, 5 , Hao Yin 1, 3 , Jie Huang 1, 3 , Yi-Juan Tan 3 , Zhen-Xing Wang 3 , Jia Cao 3 , Zheng-Zhao Liu 3 , Ping-Li Xie 6 , Ben Wu 3 , Juan Luo 3 , Hui Xie 1, 3, 4, 7, 8, 9
|
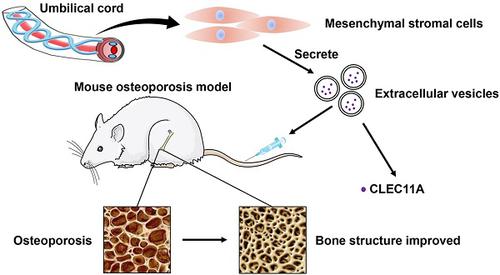
Osteoporosis and osteoporotic fractures severely compromise quality of life in elderly people and lead to early death. Human umbilical cord mesenchymal stromal cell (MSC)-derived extracellular vesicles (hucMSC-EVs) possess considerable therapeutic effects in tissue repair and regeneration. Thus, in the present study, we investigated the effects of hucMSC-EVs on primary and secondary osteoporosis and explored the underlying mechanisms.
Methods: hucMSCs were isolated and cultured. EVs were obtained from the conditioned medium of hucMSCs and determined by using transmission electron microscopy, dynamic light scattering and Western Blot analyses. The effects of hucMSC-EVs on ovariectomy-induced postmenopausal osteoporosis and tail suspension-induced hindlimb disuse osteoporosis in mouse models were assessed by using microcomputed tomography, biomechanical, histochemical and immunohistochemical, as well as histomorphometric analyses. Proteomic analysis was applied between hucMSC-EVs and hucMSCs to screen the candidate proteins that mediate hucMSC-EVs function. The effects of hucMSC-EVs on osteogenic and adipogenic differentiation of bone marrow mesenchymal stromal cells (BMSCs), and osteoclastogenesis of the macrophage cell line RAW264.7 in vitro were determined by using cytochemical staining and quantitative real-time PCR analysis. Subsequently, the roles of the key protein in hucMSC-EVs-induced regulation on BMSCs and RAW264.7 cells were evaluated.
Results: hucMSCs were able to differentiate into osteoblasts, adipocytes or chondrocytes and positively expressed CD29, CD44, CD73 and CD90, but negatively expressed CD34 and CD45. The morphological assessment revealed the typical cup- or sphere-shaped morphology of hucMSC-EVs with diameters predominantly ranging from 60 nm to 150 nm and expressed CD9, CD63, CD81 and TSG101. The systemic administration of hucMSC-EVs prevented bone loss and maintained bone strength in osteoporotic mice by enhancing bone formation, reducing marrow fat accumulation and decreasing bone resorption. Proteomic analysis showed that the potently pro-osteogenic protein, CLEC11A (C-type lectin domain family 11, member A) was very highly enriched in hucMSC-EVs. In addition, hucMSC-EVs enhanced the shift from adipogenic to osteogenic differentiation of BMSCs via delivering CLEC11A in vitro. Moreover, CLEC11A was required for the inhibitory effects of hucMSC-EVs on osteoclast formation.
Conclusion: Our results suggest that hucMSC-EVs serve as a critical regulator of bone metabolism by transferring CLEC11A and may represent a potential agent for prevention and treatment of osteoporosis.
中文翻译:

人脐带间充质基质细胞源性囊泡通过CLEC11A介导的骨代谢调节发挥有效的骨保护作用。
骨质疏松症和骨质疏松性骨折严重损害了老年人的生活质量并导致早期死亡。人脐带间充质基质细胞(MSC)衍生的细胞外囊泡(hucMSC-EVs)在组织修复和再生中具有重要的治疗作用。因此,在本研究中,我们调查了hucMSC-EV对原发性和继发性骨质疏松的影响,并探讨了其潜在机制。
方法:分离培养hucMSCs。EV是从hucMSC的条件培养基中获得的,并通过透射电子显微镜,动态光散射和Western Blot分析确定。通过使用微计算机断层扫描,生物力学,组织化学和免疫组织化学以及组织形态分析,评估了hucMSC-EV对小鼠卵巢切除术引起的绝经后骨质疏松症和尾部悬吊引起的后肢废用性骨质疏松症的影响。在hucMSC-EV和hucMSC之间应用了蛋白质组学分析,以筛选介导hucMSC-EVs功能的候选蛋白。hucMSC-EVs对骨髓间充质基质细胞(BMSCs)成骨和成脂分化以及巨噬细胞系RAW264.7的破骨细胞形成的影响通过使用细胞化学染色和定量实时PCR分析来确定体外。随后,评估了关键蛋白在hucMSC-EVs诱导的BMSCs和RAW264.7细胞调控中的作用。
结果:hucMSC能够分化为成骨细胞,脂肪细胞或软骨细胞,并且CD29,CD44,CD73和CD90呈阳性表达,而CD34和CD45呈阴性表达。形态学评估揭示了hucMSC-EV的典型杯状或球形形态,直径主要在60 nm至150 nm之间,并表达CD9,CD63,CD81和TSG101。hucMSC-EV的全身给药可通过增强骨形成,减少骨髓脂肪积聚和减少骨吸收来预防骨质疏松小鼠的骨质流失并保持骨强度。蛋白质组学分析表明,有力的促成骨蛋白CLEC11A(C型凝集素结构域家族11,成员A)在hucMSC-EV中高度富集。此外,hucMSC-EVs通过递送CLEC11A增强了BMSC从成脂分化向成骨分化的转变体外。此外,CLEC11A是hucMSC-EV对破骨细胞形成的抑制作用所必需的。
结论:我们的结果表明hucMSC-EVs通过转移CLEC11A成为骨代谢的关键调节剂,可能代表了预防和治疗骨质疏松症的潜在药物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号