当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How Strong is Hydrogen Bonding to Amide Nitrogen?
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-02-06 , DOI: 10.1002/cphc.201901104 Vladimir Y Mikshiev 1 , Alexander F Pozharskii 2 , Alexander Filarowski 3, 4 , Alexander S Novikov 1 , Alexander S Antonov 1 , Peter M Tolstoy 1 , Mikhail A Vovk 1 , Olesya V Khoroshilova 1
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-02-06 , DOI: 10.1002/cphc.201901104 Vladimir Y Mikshiev 1 , Alexander F Pozharskii 2 , Alexander Filarowski 3, 4 , Alexander S Novikov 1 , Alexander S Antonov 1 , Peter M Tolstoy 1 , Mikhail A Vovk 1 , Olesya V Khoroshilova 1
Affiliation
|
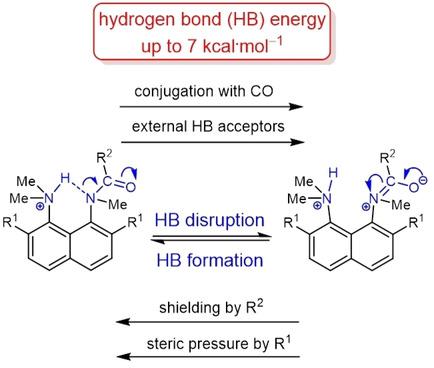
The protonation of the carboxamide nitrogen atom is an essential part of in vivo and in vitro processes (cis‐trans isomerization, amides hydrolysis etc). This phenomenon is well studied in geometrically strongly distorted amides, although there is little data concerning the protonation of undistorted amides. In the latter case, the participation of amide nitrogen in hydrogen bonding (which can be regarded as the incipient state of a proton transfer process) is less well‐studied. Thus, it would be a worthy goal to investigate the enthalpy of this interaction. We prepared and investigated a set of peri‐substituted naphthalenes containing the protonated dimethylamino group next to the amide nitrogen atom (“amide proton sponges”), which could serve as models for the study of an intramolecular hydrogen bond with the amide nitrogen atom. X‐Ray analysis, NMR spectra, basicity values as well as quantum chemical calculations revealed the existence of a hydrogen bond with the amide nitrogen, that should be attributed to the borderline between moderate and weak intramolecular hydrogen bonds (2–7 kcal ⋅ mol−1).
中文翻译:

氢键与酰胺氮的结合力有多强?
羧酰胺氮原子的质子化是体内和体外过程(顺反异构体,酰胺水解等)的重要组成部分。尽管很少有关于未扭曲酰胺质子化的数据,但在几何上高度扭曲的酰胺中对此现象进行了很好的研究。在后一种情况下,对酰胺氮在氢键中的参与(可以认为是质子转移过程的初始状态)的研究较少。因此,研究这种相互作用的焓将是一个有价值的目标。我们准备和研究了一套近郊在酰胺氮原子旁边包含质子化的二甲基氨基的预取代萘(“酰胺质子海绵”),可以用作研究与酰胺氮原子的分子内氢键的模型。X射线分析,NMR谱,碱度值以及量子化学计算揭示了氢键与酰胺氮,即应中度和弱的分子内氢键之间归因于边界线(2-7千卡⋅摩尔的存在- 1)。
更新日期:2020-02-06
中文翻译:

氢键与酰胺氮的结合力有多强?
羧酰胺氮原子的质子化是体内和体外过程(顺反异构体,酰胺水解等)的重要组成部分。尽管很少有关于未扭曲酰胺质子化的数据,但在几何上高度扭曲的酰胺中对此现象进行了很好的研究。在后一种情况下,对酰胺氮在氢键中的参与(可以认为是质子转移过程的初始状态)的研究较少。因此,研究这种相互作用的焓将是一个有价值的目标。我们准备和研究了一套近郊在酰胺氮原子旁边包含质子化的二甲基氨基的预取代萘(“酰胺质子海绵”),可以用作研究与酰胺氮原子的分子内氢键的模型。X射线分析,NMR谱,碱度值以及量子化学计算揭示了氢键与酰胺氮,即应中度和弱的分子内氢键之间归因于边界线(2-7千卡⋅摩尔的存在- 1)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号