当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Characterization of Fe3O4/Carbon Dot Supported MnO2 Nanoparticles for the Controlled Oxidation of Benzyl Alcohols
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-01-20 , DOI: 10.1002/slct.201903706 Edwin Prathibha 1 , Rajmohan Rangasamy 1 , Arunasalam Sridhar 1 , Kannappan Lakshmi 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-01-20 , DOI: 10.1002/slct.201903706 Edwin Prathibha 1 , Rajmohan Rangasamy 1 , Arunasalam Sridhar 1 , Kannappan Lakshmi 1
Affiliation
|
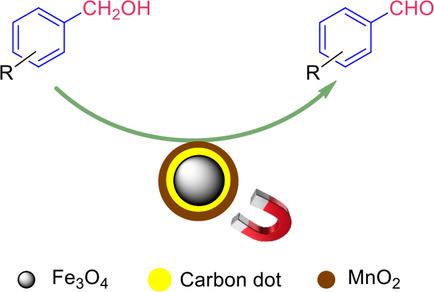
In recent past, magnetic catalyst has gained much attention because it can be easily separated from the reaction mixture. Herein we report a facile synthesis of magnetic carbon dot supported MnO2 nanoparticles for the oxidative transformation of organic compounds. In the actual synthesis, initially Fe3O4 nanoparticles were prepared by microwave assisted method and carbon dots were prepared instantaneously from glucose by simple incineration under closed condition. The synthesized carbon dots were coated over iron oxide nanoparticles via sonochemical deposition method. Further, magnetic carbon dot supported MnO2 nanocomposite was synthesized by conventional homogeneous precipitation using potassium permanganate. The synthesized nanocomposite material was characterized using FT‐IR, SEM, TEM, XRD and VSM techniques. The catalytic efficiency of the nanomaterial was successfully demonstrated for the oxidation of benzyl alcohols to benzaldehydes. The advantages of this method include, controlled oxidation of alcohols to aldehydes with broad substrate scope, the use of molecular oxygen as a green oxidant and beyond that a facile magnetic separation of the catalyst material. Moreover, the catalyst can be recycled for 5 times without much loss in its efficiency.
中文翻译:

Fe3O4 /碳点负载的MnO2纳米粒子的合成及表征,用于苄醇的可控氧化
近年来,磁性催化剂由于易于从反应混合物中分离而备受关注。在本文中,我们报道了磁性碳点支撑的MnO 2纳米颗粒的简便合成,用于有机化合物的氧化转化。在实际的合成中,最初是通过微波辅助方法制备的Fe 3 O 4纳米颗粒,然后在封闭条件下通过简单的焚烧由葡萄糖即刻制备碳点。通过声化学沉积法将合成的碳点涂覆在氧化铁纳米颗粒上。此外,磁性碳点负载的MnO 2使用高锰酸钾通过常规均相沉淀法合成纳米复合材料。使用FT-IR,SEM,TEM,XRD和VSM技术对合成的纳米复合材料进行了表征。纳米材料的催化效率已成功证明了将苄醇氧化为苯甲醛。该方法的优点包括:在较宽的底物范围内将醇受控氧化为醛;使用分子氧作为绿色氧化剂;此外,该方法还易于磁分离催化剂材料。而且,该催化剂可以循环使用5次,而不会损失很多效率。
更新日期:2020-01-21
中文翻译:

Fe3O4 /碳点负载的MnO2纳米粒子的合成及表征,用于苄醇的可控氧化
近年来,磁性催化剂由于易于从反应混合物中分离而备受关注。在本文中,我们报道了磁性碳点支撑的MnO 2纳米颗粒的简便合成,用于有机化合物的氧化转化。在实际的合成中,最初是通过微波辅助方法制备的Fe 3 O 4纳米颗粒,然后在封闭条件下通过简单的焚烧由葡萄糖即刻制备碳点。通过声化学沉积法将合成的碳点涂覆在氧化铁纳米颗粒上。此外,磁性碳点负载的MnO 2使用高锰酸钾通过常规均相沉淀法合成纳米复合材料。使用FT-IR,SEM,TEM,XRD和VSM技术对合成的纳米复合材料进行了表征。纳米材料的催化效率已成功证明了将苄醇氧化为苯甲醛。该方法的优点包括:在较宽的底物范围内将醇受控氧化为醛;使用分子氧作为绿色氧化剂;此外,该方法还易于磁分离催化剂材料。而且,该催化剂可以循环使用5次,而不会损失很多效率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号