当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,4‐Disubstituted‐1,2,3‐triazole GABA Analogues: Synthesis, In Vitro Evaluation, Quantum QSAR and Molecular Docking against Pseudomonas fluorescens GABA‐AT
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-01-20 , DOI: 10.1002/slct.201901485 Lucero Díaz‐Peralta 1 , Rodrigo Said Razo‐Hernández 2 , Nina Pastor 2 , Ángel Santiago 2 , Juan Alberto Guevara‐Salazar 3 , Mario Fernández‐Zertuche 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-01-20 , DOI: 10.1002/slct.201901485 Lucero Díaz‐Peralta 1 , Rodrigo Said Razo‐Hernández 2 , Nina Pastor 2 , Ángel Santiago 2 , Juan Alberto Guevara‐Salazar 3 , Mario Fernández‐Zertuche 1
Affiliation
|
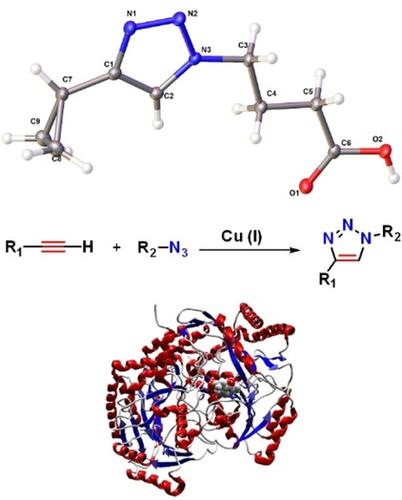
We report the synthesis of a new series of γ‐aminobutyric acid (GABA) analogues where the nitrogen at the ɣ‐position is contained in a 1,4‐disubstituted‐1,2,3‐triazole ring system. The triazole ring system was assembled by the Cu(I)‐catalyzed alkyne‐azide 1,3‐dipolar cycloaddition (CuACC) protocol. We have identified two active compounds with 43 and 59 % inhibition respectively. A descriptive quantum QSAR study was carried out to correlate this activity with the structural changes in the GABA scaffold. The inhibitory activity of the compounds is related to the electronic properties of the triazole ring and substituents. The interaction with P. fluorescens GABA‐aminotransferase (GABA‐AT) was evaluated by molecular docking, using a homology model of the biological target. One compound showed the best interaction energy, the thiophene‐ substituted triazole 26 c, with a value that correlates well with the experimental inhibition results. The triazole ring is determinant for a proper orientation for interaction with the GABA‐AT enzyme. In addition, the molecular docking revealed the importance of a hydrophobic pocket near the PLP prosthetic group and how this pocket can be used to improve the inhibitory activity of GABA analogues. Human GABA‐AT molecular docking studies of these analogues showed their great potential as competitive inhibitors of this enzyme.
中文翻译:

1,4-二取代-1,2,3-三唑GABA类似物:荧光假单胞菌GABA-AT的合成,体外评估,量子QSAR和分子对接
我们报告了一系列新的γ-氨基丁酸(GABA)类似物的合成,其中在γ-位的氮包含在1,4-二取代-1,2,3-三唑环系统中。三唑环系统是由Cu(I)催化的炔叠氮1,3-偶极环加成(CuACC)方案组装而成的。我们已鉴定出两种抑制率分别为43%和59%的活性化合物。进行了描述性量子QSAR研究,以将该活性与GABA支架中的结构变化相关联。化合物的抑制活性与三唑环和取代基的电子性质有关。与荧光假单胞菌的相互作用使用生物靶标的同源性模型,通过分子对接评估了GABA-氨基转移酶(GABA-AT)。一种化合物显示出最佳的相互作用能,噻吩取代的三唑26 c,其值与实验抑制结果密切相关。三唑环决定了与GABA-AT酶相互作用的正确方向。另外,分子对接揭示了在PLP假体附近的疏水口袋的重要性,以及如何使用该口袋改善GABA类似物的抑制活性。这些类似物的人类GABA-AT分子对接研究表明,它们作为该酶的竞争性抑制剂具有巨大潜力。
更新日期:2020-01-21
中文翻译:

1,4-二取代-1,2,3-三唑GABA类似物:荧光假单胞菌GABA-AT的合成,体外评估,量子QSAR和分子对接
我们报告了一系列新的γ-氨基丁酸(GABA)类似物的合成,其中在γ-位的氮包含在1,4-二取代-1,2,3-三唑环系统中。三唑环系统是由Cu(I)催化的炔叠氮1,3-偶极环加成(CuACC)方案组装而成的。我们已鉴定出两种抑制率分别为43%和59%的活性化合物。进行了描述性量子QSAR研究,以将该活性与GABA支架中的结构变化相关联。化合物的抑制活性与三唑环和取代基的电子性质有关。与荧光假单胞菌的相互作用使用生物靶标的同源性模型,通过分子对接评估了GABA-氨基转移酶(GABA-AT)。一种化合物显示出最佳的相互作用能,噻吩取代的三唑26 c,其值与实验抑制结果密切相关。三唑环决定了与GABA-AT酶相互作用的正确方向。另外,分子对接揭示了在PLP假体附近的疏水口袋的重要性,以及如何使用该口袋改善GABA类似物的抑制活性。这些类似物的人类GABA-AT分子对接研究表明,它们作为该酶的竞争性抑制剂具有巨大潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号