当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Insight into the Interaction between Camptothecin and Acyclic Cucurbit[4]urils as Efficient Nanocontainers in Comparison with Cucurbit[7]uril: Molecular Docking and Molecular Dynamics Simulation.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-01-16 , DOI: 10.1021/acs.jcim.9b01087 Nasim Ahmadian 1 , Faramarz Mehrnejad 1 , Mehriar Amininasab 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-01-16 , DOI: 10.1021/acs.jcim.9b01087 Nasim Ahmadian 1 , Faramarz Mehrnejad 1 , Mehriar Amininasab 2
Affiliation
|
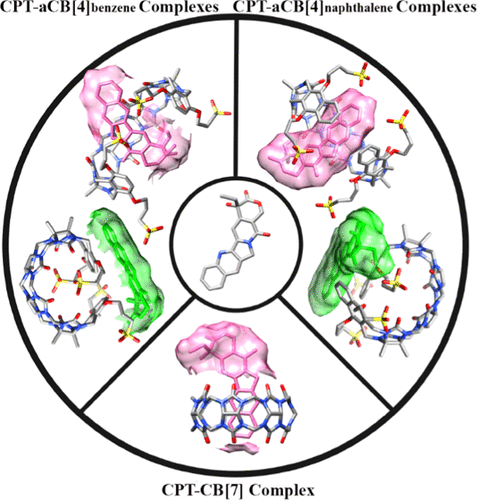
Cucurbit[n]urils (CB[n], n = 5, 6, 7, 8, 10, 14) and their derivatives due to the hydrophobic cavities and polar carbonyl portals have been considerably explored for their potential uses as drug delivery systems. It is important to understand how these macrocyclic compounds interact with guests. Camptothecin (CPT), as a natural alkaloid, is a topoisomerase inhibitor with antitumor activity against breast, pancreas, and lung cancers. The application of this drug in cancer therapy is restricted due to its low aqueous solubility and high toxicity. Recently, the complex formation between the cucurbit[7]uril (CB[7])/acyclic cucurbit[4]uril (aCB[4]) nanocontainers and CPT have been evaluated to overcome the potential drawbacks of the related drug. Herein, using computational methods, we identified the interaction mechanism of CPT with CB[7]/aCB[4]s, which consist of benzene and naphthalene sidewalls (aCB[4]benzene and aCB[4]naphthalene, respectively) since the experimental approaches have not completely provided information at the molecular level. Our molecular docking and molecular dynamics (MD) simulations show that CB[7] and its two acyclic derivatives form stable inclusion complexes with CPT especially through hydrophobic interactions. We also found that aCB[4]s with the aromatic sidewalls can attach to CPT through π-π interactions. This investigation highlights aCB[4]s due to the structural properties and flexible nature as better nanocontainers for controlled release delivery of pharmaceutical agents in comparison with the CB[7] nanocontainer.
中文翻译:

与喜树碱[7] uril相比,喜树碱与无环葫芦[4] urils作为高效纳米容器之间相互作用的分子洞察:分子对接和分子动力学模拟。
由于疏水腔和极性羰基门户,由于葫芦[n] urils(CB [n],n = 5、6、7、8、10、14)及其衍生物,它们作为药物递送系统的潜在用途已得到了广泛研究。重要的是要了解这些大环化合物如何与客体相互作用。喜树碱(CPT)是一种天然生物碱,是一种拓扑异构酶抑制剂,对乳腺癌,胰腺癌和肺癌具有抗肿瘤活性。由于该药物的低水溶性和高毒性,限制了其在癌症治疗中的应用。最近,已经评估了葫芦[7]尿素(CB [7])/无环葫芦[4]尿素(aCB [4])纳米容器与CPT之间的复合物形成,以克服相关药物的潜在缺点。在这里,我们使用计算方法确定了CPT与CB [7] / aCB [4] s的相互作用机理,由苯和萘的侧壁(分别为aCB [4]苯和aCB [4]萘)组成,因为实验方法尚未完全提供分子水平的信息。我们的分子对接和分子动力学(MD)模拟表明,CB [7]及其两个无环衍生物与CPT形成稳定的包合物,尤其是通过疏水相互作用。我们还发现,具有芳香族侧壁的aCB [4] s可以通过π-π相互作用连接到CPT。这项研究突出了aCB [4] s的结构特性和柔韧性,与CB [7]纳米容器相比,aCB [4]作为更好的纳米容器可用于药物的控释递送。分别),因为实验方法尚未完全提供分子水平的信息。我们的分子对接和分子动力学(MD)模拟表明,CB [7]及其两个无环衍生物与CPT形成稳定的包合物,尤其是通过疏水相互作用。我们还发现,具有芳香族侧壁的aCB [4] s可以通过π-π相互作用连接到CPT。这项研究突出了aCB [4] s的结构特性和柔韧性,与CB [7]纳米容器相比,aCB [4] s是更好的纳米容器,用于药物的控释递送。分别),因为实验方法尚未完全提供分子水平的信息。我们的分子对接和分子动力学(MD)模拟表明,CB [7]及其两个无环衍生物与CPT形成稳定的包合物,尤其是通过疏水相互作用。我们还发现,具有芳香族侧壁的aCB [4] s可以通过π-π相互作用连接到CPT。这项研究突出了aCB [4] s的结构特性和柔韧性,与CB [7]纳米容器相比,aCB [4]作为更好的纳米容器可用于药物的控释递送。我们的分子对接和分子动力学(MD)模拟表明,CB [7]及其两个无环衍生物与CPT形成稳定的包合物,尤其是通过疏水相互作用。我们还发现,具有芳香族侧壁的aCB [4] s可以通过π-π相互作用连接到CPT。这项研究突出了aCB [4] s的结构特性和柔韧性,与CB [7]纳米容器相比,aCB [4]作为更好的纳米容器可用于药物的控释递送。我们的分子对接和分子动力学(MD)模拟表明,CB [7]及其两个无环衍生物与CPT形成稳定的包合物,尤其是通过疏水相互作用。我们还发现,具有芳香族侧壁的aCB [4] s可以通过π-π相互作用连接到CPT。这项研究突出了aCB [4] s的结构特性和柔韧性,与CB [7]纳米容器相比,aCB [4]作为更好的纳米容器可用于药物的控释递送。
更新日期:2020-01-16
中文翻译:

与喜树碱[7] uril相比,喜树碱与无环葫芦[4] urils作为高效纳米容器之间相互作用的分子洞察:分子对接和分子动力学模拟。
由于疏水腔和极性羰基门户,由于葫芦[n] urils(CB [n],n = 5、6、7、8、10、14)及其衍生物,它们作为药物递送系统的潜在用途已得到了广泛研究。重要的是要了解这些大环化合物如何与客体相互作用。喜树碱(CPT)是一种天然生物碱,是一种拓扑异构酶抑制剂,对乳腺癌,胰腺癌和肺癌具有抗肿瘤活性。由于该药物的低水溶性和高毒性,限制了其在癌症治疗中的应用。最近,已经评估了葫芦[7]尿素(CB [7])/无环葫芦[4]尿素(aCB [4])纳米容器与CPT之间的复合物形成,以克服相关药物的潜在缺点。在这里,我们使用计算方法确定了CPT与CB [7] / aCB [4] s的相互作用机理,由苯和萘的侧壁(分别为aCB [4]苯和aCB [4]萘)组成,因为实验方法尚未完全提供分子水平的信息。我们的分子对接和分子动力学(MD)模拟表明,CB [7]及其两个无环衍生物与CPT形成稳定的包合物,尤其是通过疏水相互作用。我们还发现,具有芳香族侧壁的aCB [4] s可以通过π-π相互作用连接到CPT。这项研究突出了aCB [4] s的结构特性和柔韧性,与CB [7]纳米容器相比,aCB [4]作为更好的纳米容器可用于药物的控释递送。分别),因为实验方法尚未完全提供分子水平的信息。我们的分子对接和分子动力学(MD)模拟表明,CB [7]及其两个无环衍生物与CPT形成稳定的包合物,尤其是通过疏水相互作用。我们还发现,具有芳香族侧壁的aCB [4] s可以通过π-π相互作用连接到CPT。这项研究突出了aCB [4] s的结构特性和柔韧性,与CB [7]纳米容器相比,aCB [4] s是更好的纳米容器,用于药物的控释递送。分别),因为实验方法尚未完全提供分子水平的信息。我们的分子对接和分子动力学(MD)模拟表明,CB [7]及其两个无环衍生物与CPT形成稳定的包合物,尤其是通过疏水相互作用。我们还发现,具有芳香族侧壁的aCB [4] s可以通过π-π相互作用连接到CPT。这项研究突出了aCB [4] s的结构特性和柔韧性,与CB [7]纳米容器相比,aCB [4]作为更好的纳米容器可用于药物的控释递送。我们的分子对接和分子动力学(MD)模拟表明,CB [7]及其两个无环衍生物与CPT形成稳定的包合物,尤其是通过疏水相互作用。我们还发现,具有芳香族侧壁的aCB [4] s可以通过π-π相互作用连接到CPT。这项研究突出了aCB [4] s的结构特性和柔韧性,与CB [7]纳米容器相比,aCB [4]作为更好的纳米容器可用于药物的控释递送。我们的分子对接和分子动力学(MD)模拟表明,CB [7]及其两个无环衍生物与CPT形成稳定的包合物,尤其是通过疏水相互作用。我们还发现,具有芳香族侧壁的aCB [4] s可以通过π-π相互作用连接到CPT。这项研究突出了aCB [4] s的结构特性和柔韧性,与CB [7]纳米容器相比,aCB [4]作为更好的纳米容器可用于药物的控释递送。











































 京公网安备 11010802027423号
京公网安备 11010802027423号