当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bis(phosphine)hydridorhodacarborane Derivatives of 1,1'-Bis(ortho-carborane) and Their Catalysis of Alkene Isomerization and the Hydrosilylation of Acetophenone.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-01-16 , DOI: 10.1021/acs.inorgchem.9b03351 Antony P Y Chan 1 , John A Parkinson 2 , Georgina M Rosair 1 , Alan J Welch 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-01-16 , DOI: 10.1021/acs.inorgchem.9b03351 Antony P Y Chan 1 , John A Parkinson 2 , Georgina M Rosair 1 , Alan J Welch 1
Affiliation
|
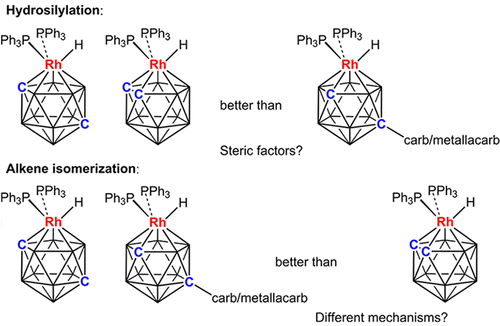
Deprotonation of [7-(1'-closo-1',2'-C2B10H11)-nido-7,8-C2B9H11]- and reaction with [Rh(PPh3)3Cl] results in isomerization of the metalated cage and the formation of [8-(1'-closo-1',2'-C2B10H11)-2-H-2,2-(PPh3)2-closo-2,1,8-RhC2B9H10] (1). Similarly, deprotonation/metalation of [8'-(7-nido-7,8-C2B9H11)-2'-(p-cymene)-closo-2',1',8'-RuC2B9H10]- and [8'-(7-nido-7,8-C2B9H11)-2'-Cp*-closo-2',1',8'-CoC2B9H10]- affords [8-{8'-2'-(p-cymene)-closo-2',1',8'-RuC2B9H10}-2-H-2,2-(PPh3)2-closo-2,1,8-RhC2B9H10] (2) and [8-(8'-2'-Cp*-closo-2',1',8'-CoC2B9H10)-2-H-2,2-(PPh3)2-closo-2,1,8-RhC2B9H10] (3), respectively, as diastereoisomeric mixtures. The performances of compounds 1-3 as catalysts in the isomerization of 1-hexene and in the hydrosilylation of acetophenone are compared with those of the known single-cage species [3-H-3,3-(PPh3)2-closo-3,1,2-RhC2B9H11] (I) and [2-H-2,2-(PPh3)2-closo-2,1,12-RhC2B9H11] (V), the last two compounds also being the subjects of 103Rh NMR spectroscopic studies, the first such investigations of rhodacarboranes. In alkene isomerization all the 2,1,8- or 2,1,12-RhC2B9 species (1-3, V) outperform the 3,1,2-RhC2B9 compound I, while for hydrosilylation the single-cage compounds I and V are better catalysts than the double-cage species 1-3.
中文翻译:

1,1'-双(原-碳烷)的双(膦)氢化硼碳硼烷衍生物及其催化烯烃异构化和苯乙酮的氢化硅烷化反应。
[7-(1'-closo-1',2'-C2B10H11)-nido-7,8-C2B9H11]-的质子化和与[Rh(PPh3)3Cl]的反应导致金属笼的异构化并形成[8-(1'-closo-1',2'-C2B10H11)-2-H-2,2-(PPh3)2-closo-2,1,8-RhC2B9H10](1)。类似地,[8'-(7-nido-7,8-C2B9H11)-2'-(对-异丙基)-closo-2',1',8'-RuC2B9H10]-和[8'- (7-nido-7,8-C2B9H11)-2'-Cp * -closo-2',1',8'-CoC2B9H10]-提供[8- {8'-2'-(p-cymene)-closo -2',1',8'-RuC2B9H10} -2-H-2,2-(PPh3)2-closo-2,1,8-RhC2B9H10](2)和[8-(8'-2'- Cp * -closo-2',1',8'-CoC2B9H10)-2-H-2,2-(PPh3)2-closo-2,1,8-RhC2B9H10](3),分别为非对映异构体混合物。将化合物1-3作为催化剂在1-己烯的异构化和苯乙酮的氢化硅烷化中的性能与已知的单笼型化合物[3-H-3,3-(PPh3)2-closo-3 ,1,2,RhC2B9H11](I)和[2-H-2,2-(PPh3)2-closo-2,1,12-RhC2B9H11](V),最后两种化合物也是103Rh NMR的主题光谱学研究中,首次对杜鹃碳烷进行了此类研究。在烯烃异构化中,所有2,1,8-或2,1,12-RhC2B9物种(1-3,V)均优于3,1,2-RhC2B9化合物I,而对于氢化硅烷化而言,单笼化合物I和V是比双笼式1-3更好的催化剂。最后两种化合物也是103Rh NMR光谱学的研究对象,其中第一类是杜鹃碳烷的研究。在烯烃异构化中,所有2,1,8-或2,1,12-RhC2B9物种(1-3,V)均优于3,1,2-RhC2B9化合物I,而对于氢化硅烷化而言,单笼化合物I和V是比双笼式1-3更好的催化剂。最后两种化合物也是103Rh NMR光谱学的研究对象,其中第一类是杜鹃碳烷的研究。在烯烃异构化中,所有2,1,8-或2,1,12-RhC2B9物种(1-3,V)均优于3,1,2-RhC2B9化合物I,而对于氢化硅烷化而言,单笼化合物I和V是比双笼式1-3更好的催化剂。
更新日期:2020-01-17
中文翻译:

1,1'-双(原-碳烷)的双(膦)氢化硼碳硼烷衍生物及其催化烯烃异构化和苯乙酮的氢化硅烷化反应。
[7-(1'-closo-1',2'-C2B10H11)-nido-7,8-C2B9H11]-的质子化和与[Rh(PPh3)3Cl]的反应导致金属笼的异构化并形成[8-(1'-closo-1',2'-C2B10H11)-2-H-2,2-(PPh3)2-closo-2,1,8-RhC2B9H10](1)。类似地,[8'-(7-nido-7,8-C2B9H11)-2'-(对-异丙基)-closo-2',1',8'-RuC2B9H10]-和[8'- (7-nido-7,8-C2B9H11)-2'-Cp * -closo-2',1',8'-CoC2B9H10]-提供[8- {8'-2'-(p-cymene)-closo -2',1',8'-RuC2B9H10} -2-H-2,2-(PPh3)2-closo-2,1,8-RhC2B9H10](2)和[8-(8'-2'- Cp * -closo-2',1',8'-CoC2B9H10)-2-H-2,2-(PPh3)2-closo-2,1,8-RhC2B9H10](3),分别为非对映异构体混合物。将化合物1-3作为催化剂在1-己烯的异构化和苯乙酮的氢化硅烷化中的性能与已知的单笼型化合物[3-H-3,3-(PPh3)2-closo-3 ,1,2,RhC2B9H11](I)和[2-H-2,2-(PPh3)2-closo-2,1,12-RhC2B9H11](V),最后两种化合物也是103Rh NMR的主题光谱学研究中,首次对杜鹃碳烷进行了此类研究。在烯烃异构化中,所有2,1,8-或2,1,12-RhC2B9物种(1-3,V)均优于3,1,2-RhC2B9化合物I,而对于氢化硅烷化而言,单笼化合物I和V是比双笼式1-3更好的催化剂。最后两种化合物也是103Rh NMR光谱学的研究对象,其中第一类是杜鹃碳烷的研究。在烯烃异构化中,所有2,1,8-或2,1,12-RhC2B9物种(1-3,V)均优于3,1,2-RhC2B9化合物I,而对于氢化硅烷化而言,单笼化合物I和V是比双笼式1-3更好的催化剂。最后两种化合物也是103Rh NMR光谱学的研究对象,其中第一类是杜鹃碳烷的研究。在烯烃异构化中,所有2,1,8-或2,1,12-RhC2B9物种(1-3,V)均优于3,1,2-RhC2B9化合物I,而对于氢化硅烷化而言,单笼化合物I和V是比双笼式1-3更好的催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号