当前位置:
X-MOL 学术
›
JAMA Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Dasatinib vs Imatinib in the Treatment of Pediatric Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Randomized Clinical Trial.
JAMA Oncology ( IF 22.5 ) Pub Date : 2020-01-16 , DOI: 10.1001/jamaoncol.2019.5868 Shuhong Shen 1 , Xiaojuan Chen 2 , Jiaoyang Cai 1 , Jie Yu 3 , Ju Gao 4 , Shaoyan Hu 5 , Xiaowen Zhai 6 , Changda Liang 7 , Xiuli Ju 8 , Hua Jiang 9 , Runming Jin 10 , Xuedong Wu 11 , Ningling Wang 12 , Xin Tian 13 , Kaili Pan 14 , Hui Jiang 15 , Lirong Sun 16 , Yongjun Fang 17 , Chi-Kong Li 18 , Qun Hu 19 , Minghua Yang 20 , Yiping Zhu 4 , Hui Zhang 9 , Chunfu Li 11 , Deqing Pei 21 , Sima Jeha 22 , Jun J Yang 23 , Cheng Cheng 21 , Jingyan Tang 1 , Xiaofan Zhu 2 , Ching-Hon Pui 22
JAMA Oncology ( IF 22.5 ) Pub Date : 2020-01-16 , DOI: 10.1001/jamaoncol.2019.5868 Shuhong Shen 1 , Xiaojuan Chen 2 , Jiaoyang Cai 1 , Jie Yu 3 , Ju Gao 4 , Shaoyan Hu 5 , Xiaowen Zhai 6 , Changda Liang 7 , Xiuli Ju 8 , Hua Jiang 9 , Runming Jin 10 , Xuedong Wu 11 , Ningling Wang 12 , Xin Tian 13 , Kaili Pan 14 , Hui Jiang 15 , Lirong Sun 16 , Yongjun Fang 17 , Chi-Kong Li 18 , Qun Hu 19 , Minghua Yang 20 , Yiping Zhu 4 , Hui Zhang 9 , Chunfu Li 11 , Deqing Pei 21 , Sima Jeha 22 , Jun J Yang 23 , Cheng Cheng 21 , Jingyan Tang 1 , Xiaofan Zhu 2 , Ching-Hon Pui 22
Affiliation
|
Importance
A randomized clinical trial is needed to determine whether the second-generation Abl-tyrosine kinase inhibitor dasatinib is more effective than the first-generation inhibitor imatinib mesylate for childhood Philadelphia chromosome-positive acute lymphoblastic leukemia (ALL).
Objective
To determine whether dasatinib given at a daily dosage of 80 mg/m2 is more effective than imatinib mesylate at a daily dosage of 300 mg/m2 to improve event-free survival of children with Philadelphia chromosome-positive ALL in the context of intensive chemotherapy without prophylactic cranial irradiation.
Design, Setting, and Participants
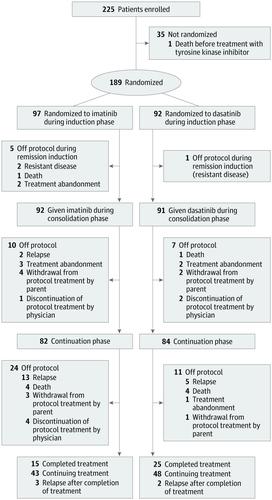
This open-label, phase 3 randomized clinical trial was conducted at 20 hospitals in China. Enrollment occurred from January 1, 2015, through September 18, 2018, and randomization was stopped on October 4, 2018, when the early stopping criterion of the trial was met. Patients aged 0 to 18 years were recruited. Of the 225 patients with the diagnosis, 35 declined participation and 1 died before treatment, leaving 189 patients available for analysis. Data were analyzed from January 1 through August 4, 2019.
Interventions
Patients were randomized to receive daily dasatinib (n = 92) or imatinib (n = 97) continuously for the entire duration of ALL therapy from the time of diagnosis made during remission induction to the end of continuation therapy.
Main Outcomes and Measures
The primary outcome was event-free survival, analyzed based on intention to treat. The secondary outcomes were relapse, death due to toxic effects, and overall survival.
Results
Among the 189 participants (136 male [72.0%]; median age, 7.8 [interquartile range (IQR), 5.2-11.3] years) and a median follow-up of 26.4 (IQR, 16.3-34.1) months, the 4-year event-free survival and overall survival rates were 71.0% (95% CI, 56.2%-89.6%) and 88.4% (95% CI, 81.3%-96.1%), respectively, in the dasatinib group and 48.9% (95% CI, 32.0%-74.5%; P = .005, log-rank test) and 69.2% (95% CI, 55.6%-86.2%; P = .04, log-rank test), respectively, in the imatinib group. The 4-year cumulative risk of any relapse was 19.8% (95% CI, 4.2%-35.4%) in the dasatinib group and 34.4% (95% CI, 15.6%-53.2%) in the imatinib group (P = .01, Gray test), whereas the 4-year cumulative risk of an isolated central nervous system relapse was 2.7% (95% CI, 0.0%-8.1%) in the dasatinib group and 8.4% (95% CI, 1.2%-15.6%) in the imatinib group (P = .06, Gray test). There were no significant differences in the frequency of severe toxic effects between the 2 treatment groups.
Conclusions and Relevance
Intensive chemotherapy including dasatinib at a dosage of 80 mg/m2 per day yielded superior results in the treatment of Philadelphia chromosome-positive ALL compared with imatinib mesylate at a dosage of 300 mg/m2 per day and provided excellent control of central nervous system leukemia without the use of prophylactic cranial irradiation.
Trial Registration
Chinese Clinical Trial Registry: ChiCTR-IPR-14005706.
更新日期:2020-03-12









































 京公网安备 11010802027423号
京公网安备 11010802027423号