当前位置:
X-MOL 学术
›
Anal. Chim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combination of stable isotope ratio data and chromatographic impurity signatures as a comprehensive concept for the profiling of highly prevalent synthetic cannabinoids and their precursors
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.aca.2020.01.029 Sascha Münster-Müller , Nicole Scheid , Ralf Zimmermann , Michael Pütz
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.aca.2020.01.029 Sascha Münster-Müller , Nicole Scheid , Ralf Zimmermann , Michael Pütz
|
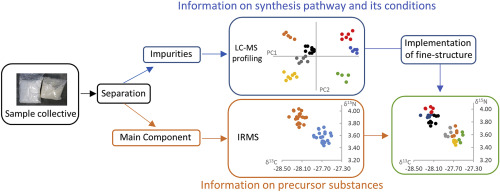
In this study, we utilized elemental analyser (EA) and gas-chromatography (GC) isotope ratio mass spectrometry (IRMS) and ultra-high-performance liquid chromatography coupled to mass spectrometry (UHPLC-MS) in a comprehensive profiling approach assessing the chromatographic impurity signatures and δ13C and δ15N isotope ratios of synthetic cannabinoids from police seizures and internet test purchases. Main target of this study was the highly prevalent synthetic cannabinoid MDMB-CHMICA (methyl (2S)-2-([1-(cyclohexylmethyl)-1H-indol-3-yl]formamido)-3,3-dimethylbutaoate). Overall, 61 powder and 118 herbal blend (also called "Spice-Products") samples were analysed using both analytical techniques and evaluated in a joint model to link samples from a common source. As a key finding, three agglomerates of Spice-product samples with similar dates of purchase were identified in the IRMS data, possibly representing larger shipments of MDMB-CHMICA, each produced with the same precursor material, successively delivered to the European market. The three agglomerates were refined into multiple sub-clusters based on the impurity profiling data, each representing an individual synthesis batch. One of the agglomerates identified in the IRMS data was found to consist two groups of four sub-clusters, respectively, with majorly different impurity profiles, demonstrating the necessity for both analytical techniques to extract the maximum amount of information from a limited sample pool. Additionally, 31 samples containing the recently surfaced synthetic cannabinoid Cumyl-PeGaClone (5-pentyl-2-(2-phenylpropan-2-yl)-2,5-dihydro-1H-pyrido[4,3-b]indol-1-one) were analysed for their and δ13C and δ15N isotope ratios to put the isotopic data recorded for MDMB-CHMNICA in a more global perspective. Three building blocks of precursor chemicals (indole, tert-leucine, cumylamine) potentially used for the synthesis of the two named synthetic cannabinoids were acquired from different global vendors and measured for their δ13C and δ15N isotope ratios to better understand variations in the isotopic composition of the synthetic cannabinoids and to trace their origin.
中文翻译:

将稳定同位素比数据和色谱杂质特征相结合,作为分析高度流行的合成大麻素及其前体的综合概念
在本研究中,我们利用元素分析仪 (EA) 和气相色谱 (GC) 同位素比质谱 (IRMS) 和超高效液相色谱与质谱联用 (UHPLC-MS) 综合分析方法来评估色谱来自警方缉获和互联网测试购买的合成大麻素的杂质特征和 δ13C 和 δ15N 同位素比率。本研究的主要目标是高度流行的合成大麻素 MDMB-CHMICA(甲基(2S)-2-([1-(环己基甲基)-1H-吲哚-3-基]甲酰胺基)-3,3-二甲基丁酸酯)。总体而言,使用两种分析技术对 61 种粉末和 118 种草药混合物(也称为“香料产品”)样品进行了分析,并在联合模型中进行了评估,以将来自共同来源的样品联系起来。作为一个关键发现,在 IRMS 数据中发现了三个具有相似购买日期的 Spice 产品样品团块,可能代表了更大的 MDMB-CHMICA 出货量,每个都使用相同的前体材料生产,相继交付到欧洲市场。根据杂质分析数据,三个附聚物被细化为多个子簇,每个子簇代表一个单独的合成批次。发现 IRMS 数据中确定的附聚物之一分别由两组四个子簇组成,分别具有主要不同的杂质分布,这表明两种分析技术都需要从有限的样本库中提取最大量的信息。此外,31 个样品含有最近浮出水面的合成大麻素 Cumyl-PeGaClone (5-pentyl-2-(2-phenylpropan-2-yl)-2,5-dihydro-1H-pyrido[4, 3-b]indol-1-one) 的 δ13C 和 δ15N 同位素比率进行了分析,以将 MDMB-CHMNICA 记录的同位素数据置于更全局的角度。从不同的全球供应商处获得了可能用于合成两种命名合成大麻素的三种前体化学品(吲哚、叔亮氨酸、枯胺)的组成部分,并测量了它们的 δ13C 和 δ15N 同位素比率,以更好地了解同位素组成的变化合成大麻素并追踪其来源。
更新日期:2020-04-01
中文翻译:

将稳定同位素比数据和色谱杂质特征相结合,作为分析高度流行的合成大麻素及其前体的综合概念
在本研究中,我们利用元素分析仪 (EA) 和气相色谱 (GC) 同位素比质谱 (IRMS) 和超高效液相色谱与质谱联用 (UHPLC-MS) 综合分析方法来评估色谱来自警方缉获和互联网测试购买的合成大麻素的杂质特征和 δ13C 和 δ15N 同位素比率。本研究的主要目标是高度流行的合成大麻素 MDMB-CHMICA(甲基(2S)-2-([1-(环己基甲基)-1H-吲哚-3-基]甲酰胺基)-3,3-二甲基丁酸酯)。总体而言,使用两种分析技术对 61 种粉末和 118 种草药混合物(也称为“香料产品”)样品进行了分析,并在联合模型中进行了评估,以将来自共同来源的样品联系起来。作为一个关键发现,在 IRMS 数据中发现了三个具有相似购买日期的 Spice 产品样品团块,可能代表了更大的 MDMB-CHMICA 出货量,每个都使用相同的前体材料生产,相继交付到欧洲市场。根据杂质分析数据,三个附聚物被细化为多个子簇,每个子簇代表一个单独的合成批次。发现 IRMS 数据中确定的附聚物之一分别由两组四个子簇组成,分别具有主要不同的杂质分布,这表明两种分析技术都需要从有限的样本库中提取最大量的信息。此外,31 个样品含有最近浮出水面的合成大麻素 Cumyl-PeGaClone (5-pentyl-2-(2-phenylpropan-2-yl)-2,5-dihydro-1H-pyrido[4, 3-b]indol-1-one) 的 δ13C 和 δ15N 同位素比率进行了分析,以将 MDMB-CHMNICA 记录的同位素数据置于更全局的角度。从不同的全球供应商处获得了可能用于合成两种命名合成大麻素的三种前体化学品(吲哚、叔亮氨酸、枯胺)的组成部分,并测量了它们的 δ13C 和 δ15N 同位素比率,以更好地了解同位素组成的变化合成大麻素并追踪其来源。









































 京公网安备 11010802027423号
京公网安备 11010802027423号