当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
LC-MS/MS method for the quantification of new psychoactive substances and evaluation of their urinary detection in humans for doping control analysis.
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2020-02-04 , DOI: 10.1002/dta.2768 Eulalia Olesti 1, 2, 3 , Jose Antonio Pascual 1, 2 , Mireia Ventura 4 , Esther Papaseit 5, 6 , Magí Farré 5, 6 , Rafael de la Torre 1, 2, 7 , Oscar J Pozo 1
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2020-02-04 , DOI: 10.1002/dta.2768 Eulalia Olesti 1, 2, 3 , Jose Antonio Pascual 1, 2 , Mireia Ventura 4 , Esther Papaseit 5, 6 , Magí Farré 5, 6 , Rafael de la Torre 1, 2, 7 , Oscar J Pozo 1
Affiliation
|
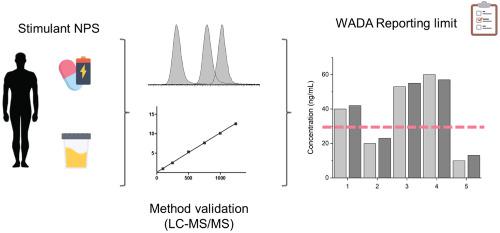
The constant legal adaptation of new psychoactive substances (NPS), challenges their evaluation in different fields. In sports, NPS are prohibited in competition with a reporting limit (RL) of 50 ng/mL for the parent compound or a metabolite. However, there is a lack of comprehensive methodologies and excretion studies for monitoring NPS. This work aims to develop an analytical methodology for the NPS quantification and to evaluate the suitability of monitoring the urinary parent stimulants after NPS misuse. A method for the quantification of 14 common NPS was developed and validated. The method was found to be linear in the range 1–1000 ng/mL, and was shown to be accurate and precise. A lowest limit of quantification (LLOQ) of 1 ng/mL was established for all analytes except for benzylpiperazine (5 ng/mL). The method was able to confirm the identity of the analytes at the LLOQ for most NPS. The methodology was applied to the quantification of the parent compound in urine samples collected from an observational study where several healthy volunteers (n ≥ 6 per drug) ingested active doses of mephedrone (MEPH), methylone (MDMC), 2,5‐dimetoxy‐4‐ethylphenetylamine (2C‐E), or 6‐(2‐aminopropyl)benzofuran (6‐APB). It was observed that for MDMC and 6‐APB, the quantification of the urinary parent drug at the current RL is a proper strategy for detecting their misuse. However, this strategy seems to be insufficient for evaluating MEPH and 2C‐E misuse. Monitoring the most abundant metabolite of MEPH (4′‐carboxy‐MEPH) and the reduction of the RL to 10 ng/mL for the 2C‐E evaluation are proposed.
中文翻译:

LC-MS / MS方法用于定量新型精神活性物质并评估其在人体中的尿检量,以进行兴奋剂控制分析。
对新型精神活性物质(NPS)的不断法律适应,对它们在不同领域的评估提出了挑战。在体育运动中,NPS禁止竞争,其母体化合物或代谢物的报告限(RL)不得超过50 ng / mL。但是,缺乏用于监测NPS的综合方法和排泄研究。这项工作旨在开发一种用于NPS定量的分析方法,并评估在NPS滥用后监测尿液母体兴奋剂的适用性。开发并验证了一种定量14种常见NPS的方法。发现该方法在1–1000 ng / mL范围内是线性的,并且被证明是准确和精确的。除苄基哌嗪(5 ng / mL)以外,所有分析物的最低定量限(LLOQ)均为1 ng / mL。对于大多数NPS,该方法能够在LLOQ处确认分析物的身份。该方法应用于从一项观察性研究中收集的尿液样品中母体化合物的定量分析,该研究中几名健康志愿者(每种药物n≥6)摄入了有效剂量的甲氧麻黄酮(MEPH),甲酮(MDMC),2,5-二甲氧基- 4-乙基苯乙胺(2C-E)或6-(2-氨基丙基)苯并呋喃(6-APB)。据观察,对于MDMC和6-APB,以当前RL定量尿母体药物是检测滥用的适当策略。但是,此策略似乎不足以评估MEPH和2C-E滥用。提出了监测MEPH(4'-羧基-MEPH)最丰富的代谢物以及将RL降低至10 ng / mL进行2C-E评价的建议。该方法应用于从一项观察性研究中收集的尿液样品中母体化合物的定量分析,该研究中几名健康志愿者(每种药物n≥6)摄入了有效剂量的甲氧麻黄酮(MEPH),甲酮(MDMC),2,5-二甲氧基- 4-乙基苯乙胺(2C-E)或6-(2-氨基丙基)苯并呋喃(6-APB)。据观察,对于MDMC和6-APB,以当前RL定量尿母体药物是检测滥用的适当策略。但是,此策略似乎不足以评估MEPH和2C-E滥用。提出了监测MEPH(4'-羧基-MEPH)最丰富的代谢物以及将RL降低至10 ng / mL进行2C-E评价的建议。该方法应用于从一项观察性研究中收集的尿液样品中母体化合物的定量分析,该研究中几名健康志愿者(每种药物n≥6)摄入了有效剂量的甲氧麻黄酮(MEPH),甲酮(MDMC),2,5-二甲氧基- 4-乙基苯乙胺(2C-E)或6-(2-氨基丙基)苯并呋喃(6-APB)。据观察,对于MDMC和6-APB,以当前RL定量尿母体药物是检测滥用的适当策略。但是,此策略似乎不足以评估MEPH和2C-E滥用。提出了监测MEPH(4'-羧基-MEPH)最丰富的代谢物以及将RL降低至10 ng / mL进行2C-E评价的建议。
更新日期:2020-02-04
中文翻译:

LC-MS / MS方法用于定量新型精神活性物质并评估其在人体中的尿检量,以进行兴奋剂控制分析。
对新型精神活性物质(NPS)的不断法律适应,对它们在不同领域的评估提出了挑战。在体育运动中,NPS禁止竞争,其母体化合物或代谢物的报告限(RL)不得超过50 ng / mL。但是,缺乏用于监测NPS的综合方法和排泄研究。这项工作旨在开发一种用于NPS定量的分析方法,并评估在NPS滥用后监测尿液母体兴奋剂的适用性。开发并验证了一种定量14种常见NPS的方法。发现该方法在1–1000 ng / mL范围内是线性的,并且被证明是准确和精确的。除苄基哌嗪(5 ng / mL)以外,所有分析物的最低定量限(LLOQ)均为1 ng / mL。对于大多数NPS,该方法能够在LLOQ处确认分析物的身份。该方法应用于从一项观察性研究中收集的尿液样品中母体化合物的定量分析,该研究中几名健康志愿者(每种药物n≥6)摄入了有效剂量的甲氧麻黄酮(MEPH),甲酮(MDMC),2,5-二甲氧基- 4-乙基苯乙胺(2C-E)或6-(2-氨基丙基)苯并呋喃(6-APB)。据观察,对于MDMC和6-APB,以当前RL定量尿母体药物是检测滥用的适当策略。但是,此策略似乎不足以评估MEPH和2C-E滥用。提出了监测MEPH(4'-羧基-MEPH)最丰富的代谢物以及将RL降低至10 ng / mL进行2C-E评价的建议。该方法应用于从一项观察性研究中收集的尿液样品中母体化合物的定量分析,该研究中几名健康志愿者(每种药物n≥6)摄入了有效剂量的甲氧麻黄酮(MEPH),甲酮(MDMC),2,5-二甲氧基- 4-乙基苯乙胺(2C-E)或6-(2-氨基丙基)苯并呋喃(6-APB)。据观察,对于MDMC和6-APB,以当前RL定量尿母体药物是检测滥用的适当策略。但是,此策略似乎不足以评估MEPH和2C-E滥用。提出了监测MEPH(4'-羧基-MEPH)最丰富的代谢物以及将RL降低至10 ng / mL进行2C-E评价的建议。该方法应用于从一项观察性研究中收集的尿液样品中母体化合物的定量分析,该研究中几名健康志愿者(每种药物n≥6)摄入了有效剂量的甲氧麻黄酮(MEPH),甲酮(MDMC),2,5-二甲氧基- 4-乙基苯乙胺(2C-E)或6-(2-氨基丙基)苯并呋喃(6-APB)。据观察,对于MDMC和6-APB,以当前RL定量尿母体药物是检测滥用的适当策略。但是,此策略似乎不足以评估MEPH和2C-E滥用。提出了监测MEPH(4'-羧基-MEPH)最丰富的代谢物以及将RL降低至10 ng / mL进行2C-E评价的建议。











































 京公网安备 11010802027423号
京公网安备 11010802027423号