Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.jechem.2020.01.011 Shiming Chen , Siglinda Perathoner , Claudio Ampelli , Hua Wei , Salvatore Abate , Bingsen Zhang , Gabriele Centi
|
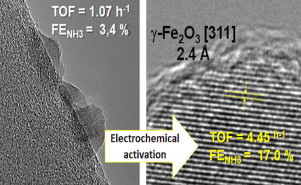
The direct electrocatalytic synthesis of ammonia from N2 and H2O by using renewable energy sources and ambient pressure/temperature operations is a breakthrough technology, which can reduce by over 90% the greenhouse gas emissions of this chemical and energy storage process. We report here an in-situ electrochemical activation method to prepare Fe2O3 CNT (iron oxide on carbon nanotubes) electrocatalysts for the direct ammonia synthesis from N2 and H2O. The in-situ electrochemical activation leads to a large increase of the ammonia formation rate and Faradaic efficiency which reach the surprising high values of 41.6 µg mgcat−1 h−1 and 17%, respectively, for an in-situ activation of 3 h, among the highest values reported so far for non-precious metal catalysts that use a continuous-flow polymer-electrolyte-membrane cell and gas-phase operations for the ammonia synthesis hemicell. The electrocatalyst was stable at least 12 h at the working conditions. Tests by switching N2 to Ar evidence that ammonia was formed from the gas-phase nitrogen. The analysis of the changes of reactivity and of the electrocatalyst characteristics as a function of the time of activation indicates a linear relationship between the ammonia formation rate and a specific XPS (X-ray-photoelectron spectroscopy) oxygen signal related to O2− in iron-oxide species. This results together with characterization data by TEM and XRD suggest that the iron species active in the direct and selective synthesis of ammonia is a maghemite-type iron oxide, and this transformation from the initial hematite is responsible for the in-situ enhancement of 3–4 times of the TOF (turnover frequency) and NH3 Faradaic efficiency. This transformation is likely related to the stabilization of the maghemite species at CNT defect sites, although for longer times of preactivation a sintering occurs with a loss of performances.
CNT (iron oxide on carbon nanotubes) electrocatalysts for the direct ammonia synthesis from N2 and H2O. The in-situ electrochemical activation leads to a large increase of the ammonia formation rate and Faradaic efficiency which reach the surprising high values of 41.6 µg mgcat−1 h−1 and 17%, respectively, for an in-situ activation of 3 h, among the highest values reported so far for non-precious metal catalysts that use a continuous-flow polymer-electrolyte-membrane cell and gas-phase operations for the ammonia synthesis hemicell. The electrocatalyst was stable at least 12 h at the working conditions. Tests by switching N2 to Ar evidence that ammonia was formed from the gas-phase nitrogen. The analysis of the changes of reactivity and of the electrocatalyst characteristics as a function of the time of activation indicates a linear relationship between the ammonia formation rate and a specific XPS (X-ray-photoelectron spectroscopy) oxygen signal related to O2− in iron-oxide species. This results together with characterization data by TEM and XRD suggest that the iron species active in the direct and selective synthesis of ammonia is a maghemite-type iron oxide, and this transformation from the initial hematite is responsible for the in-situ enhancement of 3–4 times of the TOF (turnover frequency) and NH3 Faradaic efficiency. This transformation is likely related to the stabilization of the maghemite species at CNT defect sites, although for longer times of preactivation a sintering occurs with a loss of performances.
中文翻译:

通过CNT负载的氧化铁纳米粒子的原位电化学活化,从N 2和H 2 O直接电催化合成氨中的增强性能
通过使用可再生能源和环境压力/温度操作,由N 2和H 2 O直接电催化合成氨是一项突破性技术,可以将这种化学和能量存储过程的温室气体排放量减少90%以上。我们在这里报告了一种原位电化学活化方法,用于制备Fe 2 O 3 CNT(碳纳米管上的氧化铁)电催化剂,用于直接从N 2和H 2 O合成氨。原位电化学活化导致大量增加氨生成速率和法拉第效率达到其41.6微克毫克的令人惊讶的高值猫-1 ħ -原位活化3 h分别为1%和17%,是迄今为止报道的使用连续流聚合物-电解质-膜电池和气相操作氨气的非贵金属催化剂的最高值合成半纤维素。在工作条件下,电催化剂至少稳定12小时。通过将N 2切换为Ar进行的测试证明,氨是由气相氮形成的。反应性和电催化剂特性随活化时间变化的分析表明,氨形成速率与与O 2-有关的特定XPS(X射线光电子能谱)氧信号之间呈线性关系在氧化铁中。该结果与TEM和XRD的表征数据一起表明,在氨的直接和选择性合成中有活性的铁物种是磁赤铁矿型氧化铁,从初始赤铁矿的这种转变负责原位增强3– TOF(周转频率)和NH 3法拉第效率的4倍。这种转变很可能与CNT缺陷部位的磁赤铁矿种类的稳定有关,尽管对于较长时间的预活化,烧结会发生,但性能会下降。
CNT(碳纳米管上的氧化铁)电催化剂,用于直接从N 2和H 2 O合成氨。原位电化学活化导致大量增加氨生成速率和法拉第效率达到其41.6微克毫克的令人惊讶的高值猫-1 ħ -原位活化3 h分别为1%和17%,是迄今为止报道的使用连续流聚合物-电解质-膜电池和气相操作氨气的非贵金属催化剂的最高值合成半纤维素。在工作条件下,电催化剂至少稳定12小时。通过将N 2切换为Ar进行的测试证明,氨是由气相氮形成的。反应性和电催化剂特性随活化时间变化的分析表明,氨形成速率与与O 2-有关的特定XPS(X射线光电子能谱)氧信号之间呈线性关系在氧化铁中。该结果与TEM和XRD的表征数据一起表明,在氨的直接和选择性合成中有活性的铁物种是磁赤铁矿型氧化铁,从初始赤铁矿的这种转变负责原位增强3– TOF(周转频率)和NH 3法拉第效率的4倍。这种转变很可能与CNT缺陷部位的磁赤铁矿种类的稳定有关,尽管对于较长时间的预活化,烧结会发生,但性能会下降。











































 京公网安备 11010802027423号
京公网安备 11010802027423号