当前位置:
X-MOL 学术
›
Int. J. Mass Spectrom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Precise and rapid isotopic analysis of lithium in refractory materials using NaLiBO2+ by thermal ionization mass spectrometry (TIMS)
International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.ijms.2020.116292 Radhika M. Rao , K. Sasi Bhushan , S. Jagadish Kumar , Raju Shah
International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.ijms.2020.116292 Radhika M. Rao , K. Sasi Bhushan , S. Jagadish Kumar , Raju Shah
|
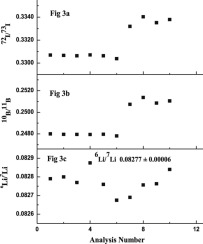
Abstract 6Li/7Li isotopic ratio was determined in lithium based compounds and refractory materials by TIMS using a novel approach. The method involved fusion of the samples such as Lithium Aluminate (LiAlO2), Lithium Titanate (Li2TiO3), Lithium Carbonate etc, with Sodium Borate flux directly on the loading filament to produce NaLiBO2+ ions at m/z 71,72 and 73 and Na2BO2+ at m/z 88 and 89. 6Li/7Li was determined by monitoring NaLiBO2+ ions at m/z 71, 72 and 73. Contributions from isotopes of boron to the ion intensity at m/z 71–73, were accounted by sequentially monitoring Na2BO2+, at m/z 88 and 89 during same analysis. L-SVEC Li2CO3was used for the initial investigations to identify the optimum composition of the analyte, sodium borate mixture required for obtaining precise 6Li/7Li ratio and the possible ion formation mechanism. Fusion of the lithium compounds with boron rich sodium borates having compositions such as Na2O·5B2O3 and Na2O·10B2O3 resulted in strong and stable molecular ion intensities for both NaLiBO2+ and Na2BO2+. Average 6Li/7Li isotopic ratio obtained for L-SVEC lithium from ten measurement using this technique was 0.08277 ± 0.00006 (S.D). Refractory compounds Li2TiO3 and LiAlO2 and lithium salts such as Li2CO3 (lithium source for the refractory compounds) and Li2B10O16 were analysed for 6Li/7Li isotopic ratio with precision of better than 0.07% (% R.S.D). Agreement between the 6Li/7Li isotopic ratio of the refractory compound and the starting material signified that there is no matrix induced mass bias. This technique not only resulted precise values for 6Li/7Li isotopic ratio but has also simplified the procedure for isotopic analysis of Li in refractory materials by circumventing the dissolution and purification step that would have been otherwise required for established mass spectrometric methods.
中文翻译:

使用 NaLiBO2+ 通过热电离质谱 (TIMS) 对耐火材料中的锂进行精确和快速的同位素分析
摘要 通过 TIMS 使用一种新方法测定了锂基化合物和耐火材料中的 6Li/7Li 同位素比。该方法包括将铝酸锂 (LiAlO2)、钛酸锂 (Li2TiO3)、碳酸锂等样品与直接在加载灯丝上的硼酸钠助熔剂熔融,以产生 m/z 71,72 和 73 的 NaLiBO2+ 离子以及 m/z 71,72 和 73 的 Na2BO2+ 离子。 m/z 88 和 89。6Li/7Li 通过监测 m/z 71、72 和 73 处的 NaLiBO2+ 离子确定。硼同位素对 m/z 71-73 处离子强度的贡献通过依次监测 Na2BO2+、在相同的分析中,在 m/z 88 和 89 处。L-SVEC Li2CO3 用于初步研究以确定分析物的最佳组成、获得精确的 6Li/7Li 比率所需的硼酸钠混合物以及可能的离子形成机制。锂化合物与具有如 Na2O·5B2O3 和 Na2O·10B2O3 等组成的富硼硼酸钠的融合导致 NaLiBO2+ 和 Na2BO2+ 的分子离子强度强而稳定。使用此技术从十次测量中获得的 L-SVEC 锂的平均 6Li/7Li 同位素比为 0.08277 ± 0.00006 (SD)。对耐火化合物 Li2TiO3 和 LiAlO2 以及锂盐(如 Li2CO3(耐火化合物的锂源)和 Li2B10O16)的 6Li/7Li 同位素比进行了分析,精度优于 0.07% (% RSD)。难熔化合物和起始材料的 6Li/7Li 同位素比之间的一致性表明不存在基质引起的质量偏差。
更新日期:2020-05-01
中文翻译:

使用 NaLiBO2+ 通过热电离质谱 (TIMS) 对耐火材料中的锂进行精确和快速的同位素分析
摘要 通过 TIMS 使用一种新方法测定了锂基化合物和耐火材料中的 6Li/7Li 同位素比。该方法包括将铝酸锂 (LiAlO2)、钛酸锂 (Li2TiO3)、碳酸锂等样品与直接在加载灯丝上的硼酸钠助熔剂熔融,以产生 m/z 71,72 和 73 的 NaLiBO2+ 离子以及 m/z 71,72 和 73 的 Na2BO2+ 离子。 m/z 88 和 89。6Li/7Li 通过监测 m/z 71、72 和 73 处的 NaLiBO2+ 离子确定。硼同位素对 m/z 71-73 处离子强度的贡献通过依次监测 Na2BO2+、在相同的分析中,在 m/z 88 和 89 处。L-SVEC Li2CO3 用于初步研究以确定分析物的最佳组成、获得精确的 6Li/7Li 比率所需的硼酸钠混合物以及可能的离子形成机制。锂化合物与具有如 Na2O·5B2O3 和 Na2O·10B2O3 等组成的富硼硼酸钠的融合导致 NaLiBO2+ 和 Na2BO2+ 的分子离子强度强而稳定。使用此技术从十次测量中获得的 L-SVEC 锂的平均 6Li/7Li 同位素比为 0.08277 ± 0.00006 (SD)。对耐火化合物 Li2TiO3 和 LiAlO2 以及锂盐(如 Li2CO3(耐火化合物的锂源)和 Li2B10O16)的 6Li/7Li 同位素比进行了分析,精度优于 0.07% (% RSD)。难熔化合物和起始材料的 6Li/7Li 同位素比之间的一致性表明不存在基质引起的质量偏差。











































 京公网安备 11010802027423号
京公网安备 11010802027423号