Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective [4+2] Annulation to the Concise Synthesis of Chiral Dihydrocarbazoles.
iScience ( IF 4.6 ) Pub Date : 2020-01-17 , DOI: 10.1016/j.isci.2020.100840 Haiyang Wang 1 , Qingdong Hu 1 , Mingxu Wang 1 , Chang Guo 1
中文翻译:

对映体合成手性二氢咔唑的对映选择性[4 + 2]。
更新日期:2020-01-17
iScience ( IF 4.6 ) Pub Date : 2020-01-17 , DOI: 10.1016/j.isci.2020.100840 Haiyang Wang 1 , Qingdong Hu 1 , Mingxu Wang 1 , Chang Guo 1
Affiliation
|
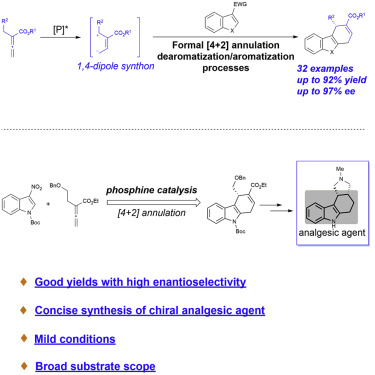
A highly efficient phosphine-catalyzed enantioselective [4 + 2] annulation of allenoates with 3-nitroindoles or 3-nitrobenzothiophenes has been developed. The protocol represents a unique dearomatization–aromatization process to access functionalized dihydrocarbazoles or dihydrodibenzothiophenes with high optical purity (up to 97% ee) under mild reaction conditions. The synthetic utility of the highly enantioselective [4 + 2] annulation enables a concise synthesis of analgesic agent.
中文翻译:

对映体合成手性二氢咔唑的对映选择性[4 + 2]。
已经开发了一种高效的膦催化的3-硝基吲哚或3-硝基苯并噻吩对烯酸酯的对映体选择性[4 + 2]环。该协议代表了独特的脱芳香化-芳香化过程,可在温和的反应条件下获得具有高光学纯度(最高ee为97%)的官能化二氢咔唑或二氢二苯并噻吩。高对映选择性[4 + 2]环空的合成实用程序可实现止痛剂的简明合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号