Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.apcatb.2020.118649 Chuanyong Jian , Wenting Hong , Qian Cai , Jing Li , Wei Liu
|
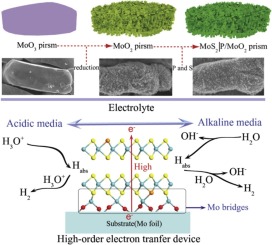
Metal sulfides (MoS2) for hydrogen evolution reaction (HER) catalysts typically suffer from inert kinetics, especially in alkaline conditions, due to its improper hydrogen adsorption/desorption capability on the basal plane S atoms. However, the hydrogen adsorption/desorption can be tuned by modulating the electron state by introducing the dopants in the basal plane of MoS2. Herein, we report a MoO2/MoS2 structure for efficient HER in alkaline and acidic electrolyte. The design of this high-performance MoO2/MoS2|P electrode is verified by density functional theory (DFT) calculation. The calculation demonstrates that the electron state of ultra-thin MoS2 can be modulated by the substitution reaction (S-O or P-O) on MoO2 surface to optimize hydrogen binding behavior and thus promote HER kinetics. Particularly, MoO2/MoS2|P (P doped) electrode exhibits an overpotential of 45 mV for 10 mA/cm2, which is a record low value among the reported transition metal dichalcogenides (TMDs) measured in the alkaline electrolyte so far.
中文翻译:

表面电子态工程增强了酸性和碱性介质中分层二硫化钼的氢释放
用于氢析出反应(HER)催化剂的金属硫化物(MoS 2)通常具有惰性动力学,尤其是在碱性条件下,由于其在基面S原子上的不适当的氢吸附/解吸能力而受到困扰。然而,可以通过在MoS 2的基面中引入掺杂剂来调节电子状态来调节氢的吸附/解吸。在本文中,我们报道了在碱性和酸性电解质中高效HER的MoO 2 / MoS 2结构。通过密度泛函理论(DFT)计算验证了该高性能MoO 2 / MoS 2 | P电极的设计。计算表明,超薄MoS 2的电子态可通过MoO 2表面上的取代反应(SO或PO)来调节H 2 O 3,以优化氢结合行为,从而促进HER动力学。特别地,MoO 2 / MoS 2 | P(P掺杂)电极在10 mA / cm 2时表现出45 mV的过电势,这是迄今为止在碱性电解质中测得的所报告的过渡金属二硫化碳(TMD)中的最低值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号