Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.apcatb.2020.118647 Meng Wang , Hongxia Liu , Jiantai Ma , Gongxuan Lu
|
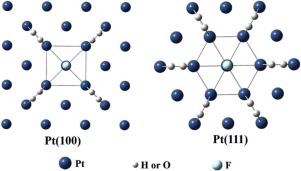
The reversible hydrogen and oxygen recombination reaction in semiconductor dispersion highly impede the yield increase of photocatalytic water splitting to hydrogen and oxygen. In order to inhibit such reverse reaction, it is necessary to reduce the degree of hydrogen and oxygen adsorption and activiation over co-catalyst on semiconductor. It is known that partial occupation of Pt sites by halogen ions with high electronegativity can decrease the adsorption and activation degree of hydrogen and oxygen molecules over Pt/TiO2, by this way, hydrogen and oxygen recombination reaction can be significantly inhibited, and the overall water splitting activity can be remarkably enhanced. Nevertheless, the detail inhibition mechanism on the reverse recombination reaction by halogen ions occupation on different Pt sites is still unclear. In the present work, by comparing the fluorine ions occupation on different Pt facets, we found that the fluorine ions occupation could decrease the numbers of adsorption sites on Pt(100) and Pt(111) surface. On Pt(100) facet, a fluorine ion occupation affect the four adjacent adsorption sites for H2 and O2 molecules adsorption, while, on Pt(111) surface, a fluorine ion occupation could affect the six adjacent adsorption sites for H2 and O2 molecules adsorption. The difference of fluorine ions occupation on the Pt(100) and Pt(111) facets led to the different adsorption strength of hydrogen and oxygen molecules, which further induced the activiation difference of hydrogen and oxygen on Pt co-catalyst. The results of density functional theory (DFT) calculation indicated that the hydrogen and oxygen adsorption energies on F/Pt(100) were higher than that on F/Pt(111) surface. The H2/O2-TPD, cyclic voltammetry and in-situ XPS experimental results also verified that the hydrogen and oxygen adsorption on F/Pt(100) surface was stronger than that of F/Pt(111) surface. The hydrogen and oxygen recombination experimental results showed that the hydrogen and oxygen recombination rates over F/Pt(100) surface was higher than that of F/Pt(111) surface. Upon light irradiation, the F/Pt(100)/TiO2 photocatalyst exhibited lower activity for overall water splitting than F/Pt(111)/TiO2 photocatalyst. Results in this paper provide a new avenue to promote seimiconductor catalyst for over-all water splitting by tuning the adsorption sites of hydrogen and oxygen on co-catalyst surface.
中文翻译:

F-预先在Pt(100)和Pt(111)助催化剂面上进行光催化分解水的活性增强
半导体分散体中可逆的氢氧重组反应严重阻碍了光催化水分裂为氢和氧的产率提高。为了抑制这种逆反应,必须降低氢和氧在半导体上的助催化剂上的吸附和活化程度。众所周知,具有高电负性的卤素离子对Pt位点的部分占据会降低氢和氧分子在Pt / TiO 2上的吸附和活化程度。这样,可以显着抑制氢和氧的再结合反应,并且可以显着提高总的水分解活性。然而,关于卤素离子在不同Pt位点处的反向重组反应的详细抑制机理仍不清楚。在目前的工作中,通过比较不同Pt面上的氟离子占有量,我们发现氟离子占有量可以减少Pt(100)和Pt(111)表面的吸附位点数量。在Pt(100)面,氟离子占用影响对于H中的四个相邻的吸附位点2和O 2层的分子吸附,同时,在Pt(111)表面,氟离子占用可能会影响用于h的六个相邻的吸附位点2和Ø 2分子吸附。氟离子在Pt(100)和Pt(111)面上的占据差异导致氢和氧分子的吸附强度不同,从而进一步引起氢和氧在Pt助催化剂上的活化差异。密度泛函理论(DFT)的计算结果表明,F / Pt(100)上的氢和氧吸附能高于F / Pt(111)上的氢和氧。H 2 / O 2-TPD,循环伏安法和原位XPS实验结果还证明,F / Pt(100)表面的氢和氧吸附强于F / Pt(111)表面。氢气和氧气的复合实验结果表明,F / Pt(100)表面的氢气和氧气的复合速率高于F / Pt(111)表面的氢气和氧气的复合速率。在光照射下,F / Pt(100)/ TiO 2光催化剂的总水分解活性低于F / Pt(111)/ TiO 2光催化剂。本文的结果为通过调节助催化剂表面上氢和氧的吸附位点提供了一种促进半导体催化剂全面分解水的新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号