当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
IKK-Mediated Regulation of the COP9 Signalosome via Phosphorylation of CSN5.
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2020-02-03 , DOI: 10.1021/acs.jproteome.9b00626 Jingzi Zhang 1 , Ruoyu Zhao 1 , Clinton Yu 2 , Christine L N Bryant 2 , Kenneth Wu 3 , Zhihong Liu 4 , Yibing Ding 1 , Yue Zhao 1 , Bin Xue 1 , Zhen-Qiang Pan 3 , Chaojun Li 1 , Lan Huang 2 , Lei Fang 1, 5
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2020-02-03 , DOI: 10.1021/acs.jproteome.9b00626 Jingzi Zhang 1 , Ruoyu Zhao 1 , Clinton Yu 2 , Christine L N Bryant 2 , Kenneth Wu 3 , Zhihong Liu 4 , Yibing Ding 1 , Yue Zhao 1 , Bin Xue 1 , Zhen-Qiang Pan 3 , Chaojun Li 1 , Lan Huang 2 , Lei Fang 1, 5
Affiliation
|
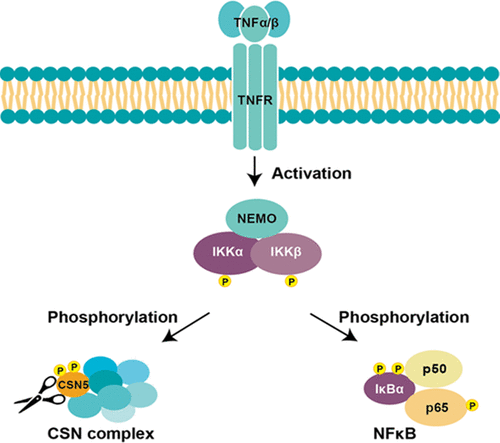
The COP9 signalosome (CSN) is an evolutionarily conserved multisubunit protein complex, which controls protein degradation through deneddylation and inactivation of cullin-RING ubiquitin E3 ligases (CRLs). Recently, the CSN complex has been linked to the NF-κB signaling pathway due to its association with the IKK complex. However, how the CSN complex is regulated in this signaling pathway remains unclear. Here, we have carried out biochemical experiments and confirmed the interaction between the CSN and IKK complexes. In addition, we have determined that overexpression of IKKα or IKKβ leads to enhanced phosphorylation of CSN5, the catalytic subunit for CSN deneddylase activity. Mutational analyses have revealed that phosphorylation at serine 201 and threonine 205 of CSN5 impairs CSN-mediated deneddylation activity in vitro. Interestingly, TNF-α treatment not only enhances the interaction between CSN and IKK but also induces an IKK-dependent phosphorylation of CSN5 at serine 201, linking CSN to TNF-α signaling through IKK. Moreover, TNF-α treatment affects the CSN interaction network globally, especially the associations of CSN with the proteasome complex, eukaryotic translation initiation factor complex, and CRL components. Collectively, our results provide new insights into IKK-mediated regulation of CSN associated with the NF-κB signaling pathway.
中文翻译:

IKK介导的CSN5磷酸化对COP9信号体的调控。
COP9信号小体(CSN)是进化上保守的多亚基蛋白质复合物,可通过cullin-ring泛素E3连接酶(CRLs)的树突化和失活控制蛋白质降解。近来,由于CSN复合物与IKK复合物的缔合,其已与NF-κB信号传导途径连接。然而,尚不清楚如何在该信号传导途径中调节CSN复合物。在这里,我们进行了生化实验并证实了CSN和IKK复合物之间的相互作用。此外,我们已经确定,IKKα或IKKβ的过度表达会导致CSN5(CSN树枝化酶活性的催化亚基)的磷酸化增强。突变分析显示,CSN5的丝氨酸201和苏氨酸205的磷酸化会削弱CSN介导的树突化活性。有趣的是 TNF-α处理不仅增强了CSN和IKK之间的相互作用,而且还诱导了IKK依赖的丝氨酸201上CSN5的磷酸化,从而通过IKK将CSN连接到TNF-α信号传导。而且,TNF-α治疗在全球范围内影响CSN相互作用网络,特别是CSN与蛋白酶体复合物,真核翻译起始因子复合物和CRL成分的关联。总的来说,我们的结果为IKK介导的与NF-κB信号通路相关的CSN调控提供了新见解。真核翻译起始因子复合物和CRL成分。总的来说,我们的结果为IKK介导的与NF-κB信号通路相关的CSN调控提供了新见解。真核翻译起始因子复合物和CRL成分。总的来说,我们的结果为IKK介导的与NF-κB信号通路相关的CSN调控提供了新见解。
更新日期:2020-02-03
中文翻译:

IKK介导的CSN5磷酸化对COP9信号体的调控。
COP9信号小体(CSN)是进化上保守的多亚基蛋白质复合物,可通过cullin-ring泛素E3连接酶(CRLs)的树突化和失活控制蛋白质降解。近来,由于CSN复合物与IKK复合物的缔合,其已与NF-κB信号传导途径连接。然而,尚不清楚如何在该信号传导途径中调节CSN复合物。在这里,我们进行了生化实验并证实了CSN和IKK复合物之间的相互作用。此外,我们已经确定,IKKα或IKKβ的过度表达会导致CSN5(CSN树枝化酶活性的催化亚基)的磷酸化增强。突变分析显示,CSN5的丝氨酸201和苏氨酸205的磷酸化会削弱CSN介导的树突化活性。有趣的是 TNF-α处理不仅增强了CSN和IKK之间的相互作用,而且还诱导了IKK依赖的丝氨酸201上CSN5的磷酸化,从而通过IKK将CSN连接到TNF-α信号传导。而且,TNF-α治疗在全球范围内影响CSN相互作用网络,特别是CSN与蛋白酶体复合物,真核翻译起始因子复合物和CRL成分的关联。总的来说,我们的结果为IKK介导的与NF-κB信号通路相关的CSN调控提供了新见解。真核翻译起始因子复合物和CRL成分。总的来说,我们的结果为IKK介导的与NF-κB信号通路相关的CSN调控提供了新见解。真核翻译起始因子复合物和CRL成分。总的来说,我们的结果为IKK介导的与NF-κB信号通路相关的CSN调控提供了新见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号