当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Potassium Nitrate Solubility in (Water + Ethanol) Mixed Solvents at Different Temperatures and Hydrochloric Acid Concentrations. Experimental Study and Modeling Using the Extended UNIQUAC Model
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-17 , DOI: 10.1021/acs.jced.9b00753 Miguel Ángel Gómez García 1, 2 , Izabela Dobrosz-Gómez 1, 3 , Juan Carlos Ojeda Toro 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-17 , DOI: 10.1021/acs.jced.9b00753 Miguel Ángel Gómez García 1, 2 , Izabela Dobrosz-Gómez 1, 3 , Juan Carlos Ojeda Toro 1, 2
Affiliation
|
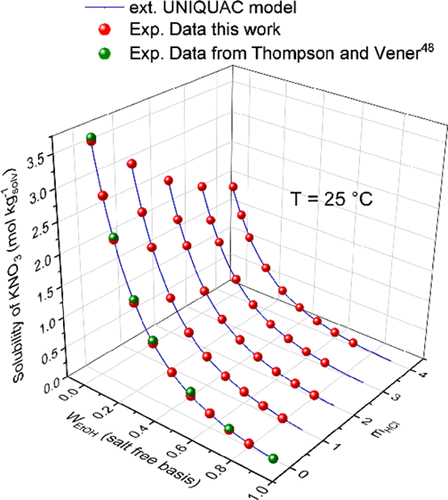
The solubility of potassium nitrate (KNO3) in water + ethanol (H2O + EtOH) mixed solvents was measured experimentally in the temperature range of 293.15 to 333.15 K and in the presence or absence of hydrochloric acid (0 ≤ HCl ≤ 4 mol kgsolv–1). The significance of these data is directly related to the simultaneous synthesis of KNO3 and HCl using a reactive crystallization process. Ethanol presented a solvent-out effect on KNO3 solubility in water. The presence of HCl in the solution also reduced its solubility. The extended UNIQUAC thermodynamic model was used to correlate the new solid–liquid phase equilibria data for the ternary (KNO3–H2O–EtOH) and quaternary (KNO3–H2O–EtOH–HCl) systems. A new set of interaction parameter values was found that allowed us to predict with a mean absolute error lower than 0.0430 mol kgsolv–1.
中文翻译:

硝酸钾在(水+乙醇)混合溶剂中在不同温度和盐酸浓度下的溶解度。使用扩展UNIQUAC模型进行实验研究和建模
在293.15至333.15 K的温度范围内以及有无盐酸(0≤HCl≤4 mol)的条件下,通过实验测量了硝酸钾(KNO 3)在水与乙醇(H 2 O + EtOH)混合溶剂中的溶解度。千克溶剂–1)。这些数据的意义直接与使用反应结晶过程同时合成KNO 3和HCl有关。乙醇对KNO 3在水中的溶解度表现出溶剂去除作用。溶液中HCl的存在也会降低其溶解度。扩展的UNIQUAC热力学模型用于关联三元(KNO 3 -H 2)的新固-液相平衡数据。O–EtOH)和四元(KNO 3 –H 2 O–EtOH–HCl)系统。发现了一组新的相互作用参数值,使我们能够以低于0.0430 mol kg solv –1的平均绝对误差进行预测。
更新日期:2020-01-17
中文翻译:

硝酸钾在(水+乙醇)混合溶剂中在不同温度和盐酸浓度下的溶解度。使用扩展UNIQUAC模型进行实验研究和建模
在293.15至333.15 K的温度范围内以及有无盐酸(0≤HCl≤4 mol)的条件下,通过实验测量了硝酸钾(KNO 3)在水与乙醇(H 2 O + EtOH)混合溶剂中的溶解度。千克溶剂–1)。这些数据的意义直接与使用反应结晶过程同时合成KNO 3和HCl有关。乙醇对KNO 3在水中的溶解度表现出溶剂去除作用。溶液中HCl的存在也会降低其溶解度。扩展的UNIQUAC热力学模型用于关联三元(KNO 3 -H 2)的新固-液相平衡数据。O–EtOH)和四元(KNO 3 –H 2 O–EtOH–HCl)系统。发现了一组新的相互作用参数值,使我们能够以低于0.0430 mol kg solv –1的平均绝对误差进行预测。











































 京公网安备 11010802027423号
京公网安备 11010802027423号