当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antibacterial Photodynamic Inactivation of Antibiotic-Resistant Bacteria and Biofilms with Nanomolar Photosensitizer Concentrations.
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2020-01-08 , DOI: 10.1021/acsinfecdis.9b00379 Carolina S Vinagreiro 1 , Amanda Zangirolami 2 , Fabio A Schaberle 1 , Sandra C C Nunes 1 , Kate C Blanco 2 , Natalia M Inada 2 , Gabriela Jorge da Silva 3 , Alberto A C C Pais 1 , Vanderlei S Bagnato 2 , Luis G Arnaut 1 , Mariette M Pereira 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2020-01-08 , DOI: 10.1021/acsinfecdis.9b00379 Carolina S Vinagreiro 1 , Amanda Zangirolami 2 , Fabio A Schaberle 1 , Sandra C C Nunes 1 , Kate C Blanco 2 , Natalia M Inada 2 , Gabriela Jorge da Silva 3 , Alberto A C C Pais 1 , Vanderlei S Bagnato 2 , Luis G Arnaut 1 , Mariette M Pereira 1
Affiliation
|
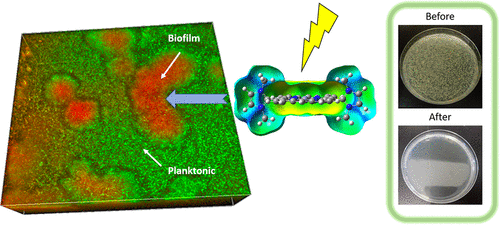
Gram-negative bacteria and bacteria in biofilms are very difficult to eradicate and are the most antibiotic-resistant bacteria. Therapeutic alternatives less susceptible to mechanisms of resistance are urgently needed to respond to an alarming increase of resistant nosocomial infections. Antibacterial photodynamic inactivation (PDI) generates oxidative stress that triggers multiple cell death mechanisms that are more difficult to counteract by bacteria. We explore PDI of multidrug-resistant bacterial strains collected from patients and show how positive charge distribution in the photosensitizer drug impacts the efficacy of inactivation. We demonstrate the relevance of size for drug diffusion in biofilms. The designed meso-imidazolyl porphyrins of small size with positive charges surrounding the macrocycle enabled the inactivation of bacteria in biofilms by 6.9 log units at 5 nM photosensitizer concentration and 5 J cm–2, which offers new opportunities to treat biofilm infections.
中文翻译:

纳米摩尔光敏剂浓度的抗细菌和生物膜的抗菌光动力失活。
革兰氏阴性细菌和生物膜中的细菌很难根除,并且是最耐抗生素的细菌。迫切需要对耐药性机制较不敏感的治疗性替代品,以应对耐药性医院感染的惊人增加。抗菌光动力失活(PDI)会产生氧化应激,从而触发多种细胞死亡机制,这些机制更难以被细菌抵消。我们探索了从患者身上收集的耐多药细菌菌株的PDI,并显示了光敏剂药物中的正电荷分布如何影响灭活的功效。我们证明了大小与生物膜中药物扩散的相关性。设计的介观小分子的咪唑基卟啉在大环周围带有正电荷,在5 nM光敏剂浓度和5 J cm –2的条件下,能够使生物膜中的细菌失活6.9 log个单位,这为治疗生物膜感染提供了新的机会。
更新日期:2020-01-08
中文翻译:

纳米摩尔光敏剂浓度的抗细菌和生物膜的抗菌光动力失活。
革兰氏阴性细菌和生物膜中的细菌很难根除,并且是最耐抗生素的细菌。迫切需要对耐药性机制较不敏感的治疗性替代品,以应对耐药性医院感染的惊人增加。抗菌光动力失活(PDI)会产生氧化应激,从而触发多种细胞死亡机制,这些机制更难以被细菌抵消。我们探索了从患者身上收集的耐多药细菌菌株的PDI,并显示了光敏剂药物中的正电荷分布如何影响灭活的功效。我们证明了大小与生物膜中药物扩散的相关性。设计的介观小分子的咪唑基卟啉在大环周围带有正电荷,在5 nM光敏剂浓度和5 J cm –2的条件下,能够使生物膜中的细菌失活6.9 log个单位,这为治疗生物膜感染提供了新的机会。









































 京公网安备 11010802027423号
京公网安备 11010802027423号