当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoredox‐Catalyzed Functionalization of Alkenes with Thiourea Dioxide: Construction of Alkyl Sulfones or Sulfonamides
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-02-18 , DOI: 10.1002/cjoc.201900505 Yuewen Li 1 , Jin‐Biao Liu 2 , Fu‐Sheng He 1 , Jie Wu 1, 3
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-02-18 , DOI: 10.1002/cjoc.201900505 Yuewen Li 1 , Jin‐Biao Liu 2 , Fu‐Sheng He 1 , Jie Wu 1, 3
Affiliation
|
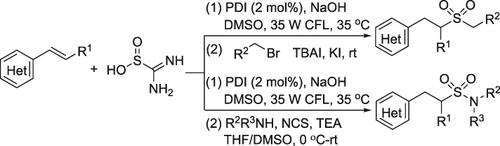
Sulfonylation of alkenes through photoredox‐catalyzed functionalization of alkenes with thiourea dioxide under visible‐light irradiation is achieved. The reaction of alkenes, thiourea dioxide and electrophiles provides a green and efficient access to alkyl sulfones and sulfonamides. A broad reaction scope is presented with good functional group compatibility and excellent regioselectivity. A plausible mechanism involving a radical addition process with sulfur dioxide radical anion (SO2‐) derived from the oxidation of sulfur dioxide anion (SO22–) is proposed, which is supported by fluorescence quenching experiments.
中文翻译:

二氧化硫脲对烯烃的光氧化还原催化功能化:烷基砜或磺酰胺的构建
在可见光照射下,通过二氧化硫脲与光氧化还原催化的烯烃官能化,实现了烯烃的磺酰化反应。烯烃,二氧化硫脲和亲电试剂的反应提供了绿色高效的烷基砜和磺酰胺通道。具有良好的官能团相容性和优异的区域选择性的反应范围广。涉及用二氧化硫自由基阴离子(SO自由基加过程的一种可能的机构2 - )从二氧化硫阴离子的氧化而得(SO 2 2-)提出,它是由荧光猝灭实验的支持。
更新日期:2020-02-18
中文翻译:

二氧化硫脲对烯烃的光氧化还原催化功能化:烷基砜或磺酰胺的构建
在可见光照射下,通过二氧化硫脲与光氧化还原催化的烯烃官能化,实现了烯烃的磺酰化反应。烯烃,二氧化硫脲和亲电试剂的反应提供了绿色高效的烷基砜和磺酰胺通道。具有良好的官能团相容性和优异的区域选择性的反应范围广。涉及用二氧化硫自由基阴离子(SO自由基加过程的一种可能的机构2 - )从二氧化硫阴离子的氧化而得(SO 2 2-)提出,它是由荧光猝灭实验的支持。











































 京公网安备 11010802027423号
京公网安备 11010802027423号