当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Iron‐Catalyzed Aminomethyloxygenative Cyclization of Hydroxy‐α‐diazoesters with N,O‐Aminals
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-02-22 , DOI: 10.1002/cjoc.201900492 Suchen Zou 1 , Tianze Zhang 1 , Siyuan Wang 1 , Hanmin Huang 1, 2
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-02-22 , DOI: 10.1002/cjoc.201900492 Suchen Zou 1 , Tianze Zhang 1 , Siyuan Wang 1 , Hanmin Huang 1, 2
Affiliation
|
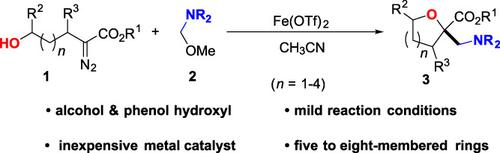
A new and efficient cyclization reaction has been developed to synthesize cyclic α,α‐disubstituted β‐amino esters via iron‐catalyzed intramolecular aminomethyloxygenative cyclization of diazo compounds with N,O‐aminal under mild reaction conditions. A broad range of hydroxy‐α‐diazoesters with different substituents and various N,O‐aminals were compatible with this protocol, affording the corresponding α,α‐disubstituted β‐amino esters bearing a five‐ to eight‐membered oxacycle in good yields.
中文翻译:

具有N,O-氨基的羟基α-重氮酸酯的铁催化氨甲基氧合环化反应
已开发出一种新的高效环化反应,通过在温和的反应条件下,通过铁催化的重氮化合物与N,O-氨基的重氮化合物的分子内氨基甲氧基加氧环化反应,合成环状α,α-二取代的β-氨基酯。各种具有不同取代基的羟基α-重氮酸酯和各种N,O-氨基缩醛与该方案兼容,从而以高收率提供了带有五至八元氧杂环的相应α,α-二取代的β-氨基酯。
更新日期:2020-02-23
中文翻译:

具有N,O-氨基的羟基α-重氮酸酯的铁催化氨甲基氧合环化反应
已开发出一种新的高效环化反应,通过在温和的反应条件下,通过铁催化的重氮化合物与N,O-氨基的重氮化合物的分子内氨基甲氧基加氧环化反应,合成环状α,α-二取代的β-氨基酯。各种具有不同取代基的羟基α-重氮酸酯和各种N,O-氨基缩醛与该方案兼容,从而以高收率提供了带有五至八元氧杂环的相应α,α-二取代的β-氨基酯。



























 京公网安备 11010802027423号
京公网安备 11010802027423号