Bone Marrow Transplantation ( IF 4.5 ) Pub Date : 2020-01-17 , DOI: 10.1038/s41409-020-0783-y Thomas Hueso 1, 2 , Thomas Gastinne 3, 4 , Sylvain Garciaz 5 , Emmanuelle Tchernonog 6 , Caroline Delette 7 , René-Olivier Casasnovas 8, 9 , Eric Durot 10 , Roch Houot 11, 12 , Benoît Tessoulin 3, 4 , Olivier Tournilhac 13 , Sandra Malak 14 , Emmanuel Gyan 15, 16 , Luc-Matthieu Fornecker 17 , Julie Abraham 18 , Baptiste Delapierre 1, 2 , Frédéric Peyrade 19 , Richard Lemal 13 , Rémy Gressin 20 , Sylvain Chantepie 1, 2 , Cécile Borel 21 , Rémy Morello 22 , Krimo Bouabdallah 23 , Ahmad Ibrahim 24 , Reda Bouabdallah 5 , Steven Le Gouill 3, 4 , Gandhi Damaj 1, 2
|
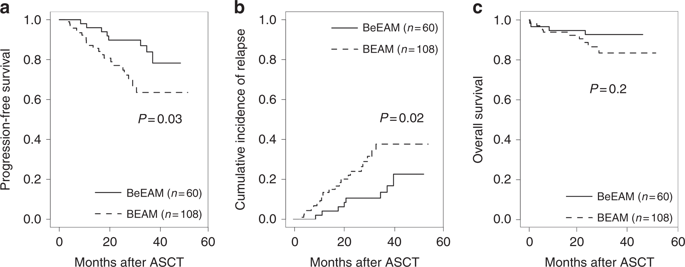
The combination of carmustine, etoposide, cytarabine, and melphalan (BEAM) as conditioning regimen prior to autologous stem-cell transplantation (ASCT) remains the standard of care for patients with mantle cell lymphoma (MCL) who are eligible for transplantation. The replacement of carmustine with bendamustine (BeEAM) was described as a promising alternative in non-Hodgkin lymphoma. The aim of this retrospective study was to compare the BeEAM with the BEAM regimen in MCL patients in the frontline setting. Sixty and 108 patients were included in the BeEAM and the BEAM groups, respectively. At 3 years, progression-free survival (PFS) was significantly higher in the BeEAM than in the BEAM group (84% [73–96] vs. 63% [51–79], p = 0.03). The overall survival was not statistically different between the two groups (p = 0.2). In multivariable analysis, BeEAM regimen remained associated with higher PFS (HR = 0.377, 95% CI, 0.146–0.970; p = 0.043). Subgroup analyses in patients treated with prior rituximab–aracytine induction alone showed that BeEAM improved the PFS compared with BEAM regimen (p = 0.04). Despite the high rate of acute renal failure KDIGO III (32%), treatment-related mortality was not increased with the BeEAM regimen. A prospective randomized trial will be necessary to confirm the beneficial effect of the BeEAM regimen in MCL patients undergoing ASCT.
中文翻译:

Bendamustine-EAM 与 BEAM 方案在一线环境中接受自体干细胞移植的套细胞淋巴瘤患者:来自淋巴瘤研究协会 (LYSA) 中心的多中心回顾性研究
卡莫司汀、依托泊苷、阿糖胞苷和美法仑 (BEAM) 的组合作为自体干细胞移植 (ASCT) 前的预处理方案仍然是适合移植的套细胞淋巴瘤 (MCL) 患者的标准治疗。用苯达莫司汀 (BeEAM) 替代卡莫司汀被描述为治疗非霍奇金淋巴瘤的有希望的替代方案。这项回顾性研究的目的是比较 BeEAM 与 BEAM 方案在一线 MCL 患者中的疗效。BeEAM 组和 BEAM 组分别有 60 名和 108 名患者。3 年时,BeEAM 组的无进展生存期 (PFS) 显着高于 BEAM 组(84% [73-96] vs. 63% [51-79],p = 0.03)。两组的总生存期无统计学差异(p = 0.2)。在多变量分析中,BeEAM 方案仍然与较高的 PFS 相关(HR = 0.377, 95% CI, 0.146–0.970; p = 0.043)。单独接受利妥昔单抗-阿拉西汀诱导治疗的患者的亚组分析表明,与 BEAM 方案相比,BeEAM 改善了 PFS ( p = 0.04)。尽管急性肾功能衰竭 KDIGO III 的发生率很高(32%),但 BeEAM 方案并未增加治疗相关死亡率。需要一项前瞻性随机试验来确认 BeEAM 方案对接受 ASCT 的 MCL 患者的有益效果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号