Bone Marrow Transplantation ( IF 4.5 ) Pub Date : 2020-01-17 , DOI: 10.1038/s41409-020-0786-8 Thomas E Hughes 1 , Lindsay Stansfield 2 , Parag Kumar 3 , Tat'Yana Worthy 4 , Xin Tian 5 , Richard W Childs 4
|
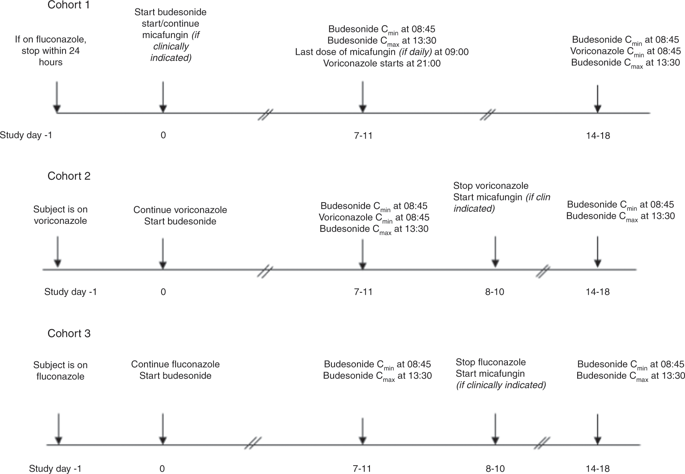
The gastrointestinal (GI) tract is commonly affected by acute and chronic graft-versus-host disease (GVHD) in patients who have undergone allogeneic hematopoietic stem cell transplantation (allo-HSCT). For patients developing GI GVHD, nonabsorbable corticosteroids such as budesonide may be used alone to reduce the risk of systemic corticosteroid toxicities or combined with systemic steroids to enhance clinical responses and to allow more rapid tapering of systemic corticosteroid doses. This prospective crossover study was conducted to evaluate what effect two commonly used antifungal agents, fluconazole, and voriconazole, would have on the trough (Cmin) and peak (Cmax) levels of budesonide in adult patients who had undergone allo-HSCT who subsequently developed clinical GI GVHD. Fifteen subjects were enrolled and nine completed the study and were evaluable. When coadministered with budesonide, voriconazole significantly increased the geometric mean of budesonide Cmin and Cmax levels by 8.52- and 6.63-fold, respectively. The cohort to evaluate the interaction with fluconazole did not meet accrual goals to reach definitive conclusions. In conclusion, this prospective study demonstrated that when patients with GI GVHD are treated with budesonide concurrently with voriconazole, the systemic concentrations of budesonide increase substantially which could increase the risk of steroid-associated toxicities.
中文翻译:

氟康唑和伏立康唑对胃肠道 GVHD 患者布地奈德血清浓度相互作用的前瞻性评价
在接受异基因造血干细胞移植 (allo-HSCT) 的患者中,胃肠道 (GI) 通常受到急性和慢性移植物抗宿主病 (GVHD) 的影响。对于发生 GI GVHD 的患者,不可吸收的皮质类固醇如布地奈德可单独使用以降低全身性皮质类固醇毒性的风险,或与全身性类固醇联合使用以增强临床反应并允许更快速地减少全身性皮质类固醇剂量。这项前瞻性交叉研究旨在评估两种常用的抗真菌药物氟康唑和伏立康唑对接受异体造血干细胞移植的成年患者布地奈德的谷 (Cmin) 和峰值 (Cmax) 水平有何影响,这些患者随后发展为临床GI GVHD。招募了 15 名受试者,其中 9 名完成了研究并进行了评估。与布地奈德合用时,伏立康唑使布地奈德 Cmin 和 Cmax 水平的几何平均值分别显着增加 8.52 倍和 6.63 倍。评估与氟康唑相互作用的队列没有达到得出明确结论的应计目标。总之,这项前瞻性研究表明,当 GI GVHD 患者同时接受布地奈德和伏立康唑治疗时,布地奈德的全身浓度显着增加,这可能会增加类固醇相关毒性的风险。评估与氟康唑相互作用的队列没有达到得出明确结论的应计目标。总之,这项前瞻性研究表明,当 GI GVHD 患者同时接受布地奈德和伏立康唑治疗时,布地奈德的全身浓度显着增加,这可能会增加类固醇相关毒性的风险。评估与氟康唑相互作用的队列没有达到得出明确结论的应计目标。总之,这项前瞻性研究表明,当 GI GVHD 患者同时接受布地奈德和伏立康唑治疗时,布地奈德的全身浓度显着增加,这可能会增加类固醇相关毒性的风险。









































 京公网安备 11010802027423号
京公网安备 11010802027423号