Nature Communications ( IF 14.7 ) Pub Date : 2020-01-17 , DOI: 10.1038/s41467-019-14219-6 Yota Yasumizu , Hasan Rajabi , Caining Jin , Tsuyoshi Hata , Sean Pitroda , Mark D. Long , Masayuki Hagiwara , Wei Li , Qiang Hu , Song Liu , Nami Yamashita , Atsushi Fushimi , Ling Kui , Mehmet Samur , Masaaki Yamamoto , Yan Zhang , Ning Zhang , Deli Hong , Takahiro Maeda , Takeo Kosaka , Kwok K. Wong , Mototsugu Oya , Donald Kufe
|
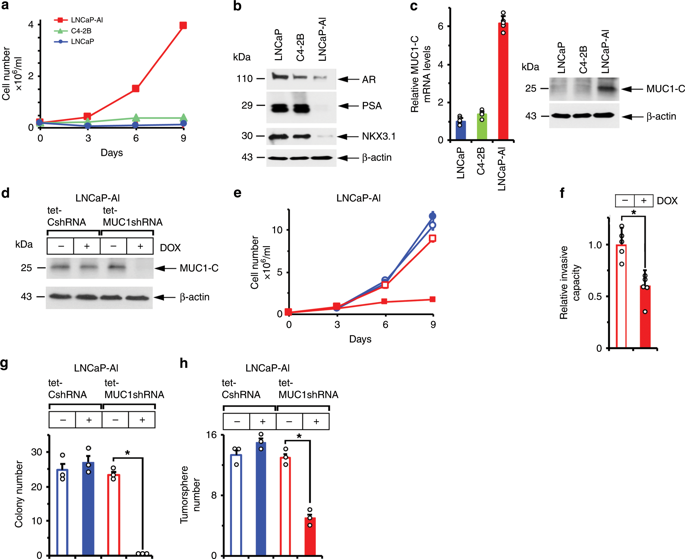
Neuroendocrine prostate cancer (NEPC) is an aggressive malignancy with no effective targeted therapies. The oncogenic MUC1-C protein is overexpressed in castration-resistant prostate cancer (CRPC) and NEPC, but its specific role is unknown. Here, we demonstrate that upregulation of MUC1-C in androgen-dependent PC cells suppresses androgen receptor (AR) axis signaling and induces the neural BRN2 transcription factor. MUC1-C activates a MYC→BRN2 pathway in association with induction of MYCN, EZH2 and NE differentiation markers (ASCL1, AURKA and SYP) linked to NEPC progression. Moreover, MUC1-C suppresses the p53 pathway, induces the Yamanaka pluripotency factors (OCT4, SOX2, KLF4 and MYC) and drives stemness. Targeting MUC1-C decreases PC self-renewal capacity and tumorigenicity, suggesting a potential therapeutic approach for CRPC and NEPC. In PC tissues, MUC1 expression associates with suppression of AR signaling and increases in BRN2 expression and NEPC score. These results highlight MUC1-C as a master effector of lineage plasticity driving progression to NEPC.
中文翻译:

MUC1-C调节谱系可塑性,驱动神经内分泌前列腺癌发展
神经内分泌前列腺癌(NEPC)是一种恶性肿瘤,没有有效的靶向治疗方法。致癌性MUC1-C蛋白在去势抵抗性前列腺癌(CRPC)和NEPC中过表达,但其具体作用尚不清楚。在这里,我们证明了雄激素依赖性PC细胞中MUC1-C的上调抑制了雄激素受体(AR)轴信号传导并诱导了神经BRN2转录因子。MUC1-C激活MYC→BRN2途径,并诱导与NEPC进展相关的MYCN,EZH2和NE分化标志物(ASCL1,AURKA和SYP)。此外,MUC1-C抑制p53途径,诱导Yamanaka多能性因子(OCT4,SOX2,KLF4和MYC)并驱动茎干。靶向MUC1-C降低了PC的自我更新能力和致瘤性,提示了CRPC和NEPC的潜在治疗方法。在PC组织中,MUC1的表达与AR信号的抑制有关,并增加BRN2的表达和NEPC评分。这些结果突出表明MUC1-C是谱系可塑性的主要效应物,推动了向NEPC的发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号