当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cryo-EM structure of the respiratory syncytial virus RNA polymerase.
Nature Communications ( IF 14.7 ) Pub Date : 2020-01-17 , DOI: 10.1038/s41467-019-14246-3 Dongdong Cao 1 , Yunrong Gao 1 , Claire Roesler 1 , Samantha Rice 1 , Paul D'Cunha 1 , Lisa Zhuang 1 , Julia Slack 1 , Mason Domke 1 , Anna Antonova 1 , Sarah Romanelli 1 , Shayon Keating 1 , Gabriela Forero 1 , Puneet Juneja 2 , Bo Liang 1
Nature Communications ( IF 14.7 ) Pub Date : 2020-01-17 , DOI: 10.1038/s41467-019-14246-3 Dongdong Cao 1 , Yunrong Gao 1 , Claire Roesler 1 , Samantha Rice 1 , Paul D'Cunha 1 , Lisa Zhuang 1 , Julia Slack 1 , Mason Domke 1 , Anna Antonova 1 , Sarah Romanelli 1 , Shayon Keating 1 , Gabriela Forero 1 , Puneet Juneja 2 , Bo Liang 1
Affiliation
|
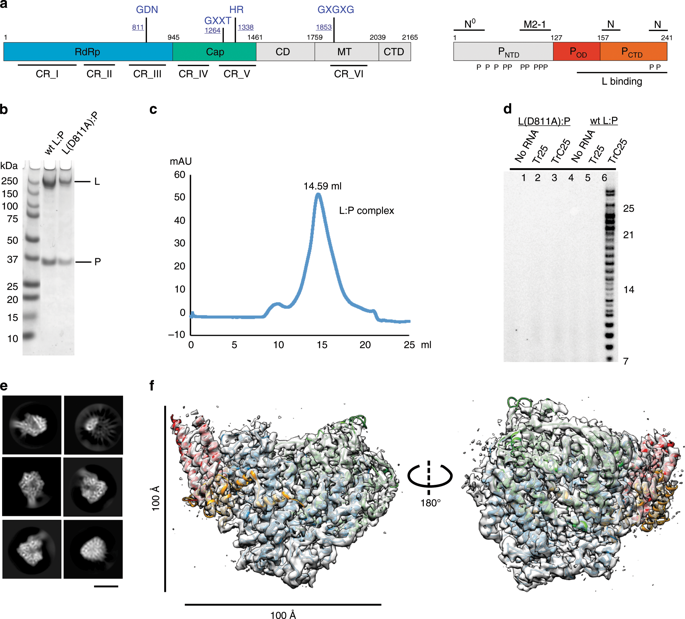
The respiratory syncytial virus (RSV) RNA polymerase, constituted of a 250 kDa large (L) protein and tetrameric phosphoprotein (P), catalyzes three distinct enzymatic activities - nucleotide polymerization, cap addition, and cap methylation. How RSV L and P coordinate these activities is poorly understood. Here, we present a 3.67 Å cryo-EM structure of the RSV polymerase (L:P) complex. The structure reveals that the RNA dependent RNA polymerase (RdRp) and capping (Cap) domains of L interact with the oligomerization domain (POD) and C-terminal domain (PCTD) of a tetramer of P. The density of the methyltransferase (MT) domain of L and the N-terminal domain of P (PNTD) is missing. Further analysis and comparison with other RNA polymerases at different stages suggest the structure we obtained is likely to be at an elongation-compatible stage. Together, these data provide enriched insights into the interrelationship, the inhibitors, and the evolutionary implications of the RSV polymerase.
中文翻译:

呼吸道合胞病毒RNA聚合酶的Cryo-EM结构。
呼吸道合胞病毒(RSV)RNA聚合酶由250 kDa大(L)蛋白和四聚体磷蛋白(P)组成,催化三种独特的酶促活性-核苷酸聚合,加帽和帽甲基化。很少了解RSV L和P如何协调这些活动。在这里,我们提出了一种RSV聚合酶(L:P)复合物的3.67Å低温-EM结构。该结构显示L的RNA依赖性RNA聚合酶(RdRp)和加帽(Cap)结构域与P的四聚体的寡聚结构域(POD)和C末端结构域(PCTD)相互作用。甲基转移酶(MT)的密度L的P结构域和P的N端结构域(PNTD)丢失。在不同阶段的进一步分析和与其他RNA聚合酶的比较表明,我们获得的结构可能处于延伸相容性阶段。一起,
更新日期:2020-01-17
中文翻译:

呼吸道合胞病毒RNA聚合酶的Cryo-EM结构。
呼吸道合胞病毒(RSV)RNA聚合酶由250 kDa大(L)蛋白和四聚体磷蛋白(P)组成,催化三种独特的酶促活性-核苷酸聚合,加帽和帽甲基化。很少了解RSV L和P如何协调这些活动。在这里,我们提出了一种RSV聚合酶(L:P)复合物的3.67Å低温-EM结构。该结构显示L的RNA依赖性RNA聚合酶(RdRp)和加帽(Cap)结构域与P的四聚体的寡聚结构域(POD)和C末端结构域(PCTD)相互作用。甲基转移酶(MT)的密度L的P结构域和P的N端结构域(PNTD)丢失。在不同阶段的进一步分析和与其他RNA聚合酶的比较表明,我们获得的结构可能处于延伸相容性阶段。一起,











































 京公网安备 11010802027423号
京公网安备 11010802027423号