Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficacy and safety of nanoparticle-albumin-bound paclitaxel compared with solvent-based taxanes for metastatic breast cancer: A meta-analysis.
Scientific Reports ( IF 3.8 ) Pub Date : 2020-01-17 , DOI: 10.1038/s41598-019-57380-0 Hwaryeon Lee 1, 2 , Sohyun Park 3 , Ji Eun Kang 4, 5 , Hee Min Lee 6, 7 , Sun Ah Kim 2, 4 , Sandy Jeong Rhie 1, 2, 3, 4, 6
Scientific Reports ( IF 3.8 ) Pub Date : 2020-01-17 , DOI: 10.1038/s41598-019-57380-0 Hwaryeon Lee 1, 2 , Sohyun Park 3 , Ji Eun Kang 4, 5 , Hee Min Lee 6, 7 , Sun Ah Kim 2, 4 , Sandy Jeong Rhie 1, 2, 3, 4, 6
Affiliation
|
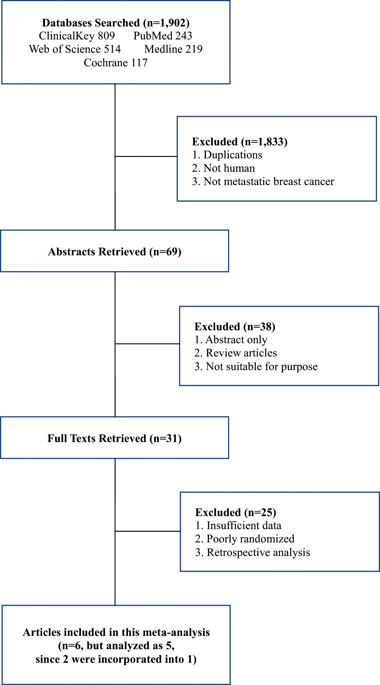
The curative effects of nanoparticle albumin-bound (nab)-paclitaxel in the first-line treatment of metastatic breast cancer (MBC) are still controversial, with even more after the removal of marketing approval of indication of bevacizumab. Five electronic databases and the related resources were searched for eligible randomized clinical trials (RCTs) without year and language restrictions to perform a meta-analysis. The studies were comparing the efficacy and safety between nab-paclitaxel chemotherapy versus solvent-based (sb)-taxanes chemotherapy such as sb-paclitaxel and docetaxel. The primary end points were overall response rate (ORR) and disease control rate (DCR). Secondary end points were progression-free survival (PFS), overall survival (OS), adverse events (AEs), and dose discontinuation rate (DDR). Five RCTs (1,554 patients) were finally identified from 1,902 studies. When compared to sb-paclitaxel, nab-paclitaxel showed significant beneficial effects in terms of ORR (OR 2.39, 95% CI 1.69-3.37, p < 0.001), DCR (OR 1.89, 95% CI 1.07-3.35, p = 0.03), and PFS (HR 0.75, 95% CI 0.62-0.90, p = 0.002). Nab-paclitaxel also showed significantly longer OS (HR 0.73, 95% CI 0.54-0.99, p = 0.04) than docetaxel. AEs and DDR were comparable between the two arms. Using nab-paclitaxel could significantly improve efficacy with comparable toxicities in the treatment of MBC.
中文翻译:

纳米颗粒白蛋白结合紫杉醇与溶剂型紫杉烷相比对转移性乳腺癌的疗效和安全性:一项荟萃分析。
纳米颗粒白蛋白结合(nab)紫杉醇一线治疗转移性乳腺癌(MBC)的疗效仍存在争议,在贝伐珠单抗适应症取消上市后更是如此。搜索了五个电子数据库和相关资源,寻找符合条件的随机临床试验(RCT),没有年份和语言限制,以进行荟萃分析。这些研究比较了白蛋白结合型紫杉醇化疗与溶剂型(sb)紫杉烷化疗(例如sb-紫杉醇和多西他赛)之间的疗效和安全性。主要终点是总体缓解率(ORR)和疾病控制率(DCR)。次要终点是无进展生存期(PFS)、总生存期(OS)、不良事件(AE)和剂量停药率(DDR)。最终从 1,902 项研究中确定了 5 项随机对照试验(1,554 名患者)。与 sb-紫杉醇相比,白蛋白结合型紫杉醇在 ORR(OR 2.39,95% CI 1.69-3.37,p < 0.001)和 DCR(OR 1.89,95% CI 1.07-3.35,p = 0.03)方面显示出显着的有益效果和 PFS(HR 0.75,95% CI 0.62-0.90,p = 0.002)。白蛋白结合型紫杉醇还表现出比多西紫杉醇显着更长的 OS(HR 0.73,95% CI 0.54-0.99,p = 0.04)。两组之间的 AE 和 DDR 相当。使用白蛋白结合型紫杉醇可显着提高治疗 MBC 的疗效,且毒性相当。
更新日期:2020-01-17
中文翻译:

纳米颗粒白蛋白结合紫杉醇与溶剂型紫杉烷相比对转移性乳腺癌的疗效和安全性:一项荟萃分析。
纳米颗粒白蛋白结合(nab)紫杉醇一线治疗转移性乳腺癌(MBC)的疗效仍存在争议,在贝伐珠单抗适应症取消上市后更是如此。搜索了五个电子数据库和相关资源,寻找符合条件的随机临床试验(RCT),没有年份和语言限制,以进行荟萃分析。这些研究比较了白蛋白结合型紫杉醇化疗与溶剂型(sb)紫杉烷化疗(例如sb-紫杉醇和多西他赛)之间的疗效和安全性。主要终点是总体缓解率(ORR)和疾病控制率(DCR)。次要终点是无进展生存期(PFS)、总生存期(OS)、不良事件(AE)和剂量停药率(DDR)。最终从 1,902 项研究中确定了 5 项随机对照试验(1,554 名患者)。与 sb-紫杉醇相比,白蛋白结合型紫杉醇在 ORR(OR 2.39,95% CI 1.69-3.37,p < 0.001)和 DCR(OR 1.89,95% CI 1.07-3.35,p = 0.03)方面显示出显着的有益效果和 PFS(HR 0.75,95% CI 0.62-0.90,p = 0.002)。白蛋白结合型紫杉醇还表现出比多西紫杉醇显着更长的 OS(HR 0.73,95% CI 0.54-0.99,p = 0.04)。两组之间的 AE 和 DDR 相当。使用白蛋白结合型紫杉醇可显着提高治疗 MBC 的疗效,且毒性相当。











































 京公网安备 11010802027423号
京公网安备 11010802027423号