Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
No Evidence of Off-label Use of Olodaterol and Indacaterol in Denmark, France, and the Netherlands: A Drug Utilization Study.
Scientific Reports ( IF 3.8 ) Pub Date : 2020-01-17 , DOI: 10.1038/s41598-019-57397-5 Cristina Rebordosa 1, 2 , Eline Houben 3 , Kristina Laugesen 4 , Ulrich Bothner 5 , Jukka Montonen 5 , Jaume Aguado 1, 2 , Jetty A Overbeek 2 , Vera Ehrenstein 4 , Joelle Asmar 6 , Laura Wallace 7 , Alicia W Gilsenan 1, 2
Scientific Reports ( IF 3.8 ) Pub Date : 2020-01-17 , DOI: 10.1038/s41598-019-57397-5 Cristina Rebordosa 1, 2 , Eline Houben 3 , Kristina Laugesen 4 , Ulrich Bothner 5 , Jukka Montonen 5 , Jaume Aguado 1, 2 , Jetty A Overbeek 2 , Vera Ehrenstein 4 , Joelle Asmar 6 , Laura Wallace 7 , Alicia W Gilsenan 1, 2
Affiliation
|
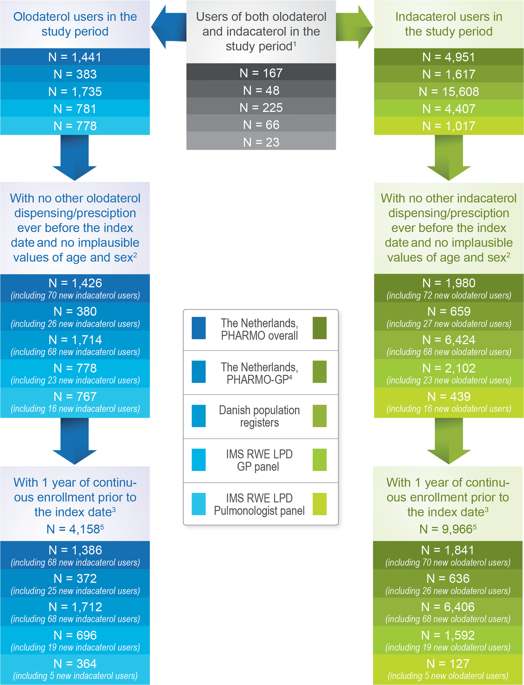
To characterize the use of olodaterol and indacaterol in clinical practice and to quantify the off-label use in asthma. Drug utilization study of new users of olodaterol or indacaterol between 2014 and 2017 in the PHARMO Database Network in the Netherlands, the Danish population registers, and the IMS Real-World Evidence Longitudinal Patient Database panels in France. On-label use was defined as use among adults with a recorded diagnosis of COPD. Off-label use was defined as use among adults with a recorded diagnosis of asthma without a recorded diagnosis of COPD or as use among patients aged ≤18 years. Potential off-label use was defined as no recorded diagnosis of either COPD or asthma. The study included 4,158 new users of olodaterol and 9,966 new users of indacaterol. Prevalence of off-label use ranged from 3.5% for both drugs to 12.4% for olodaterol and 11.9% for indacaterol. Prevalence of on-label use ranged from 47.8% to 77.7% for olodaterol and from 28.7% to 70.1% for indacaterol. The remaining new users of olodaterol and indacaterol were classified as potential off-label users, with prevalence ranging from 17.3% to 48.6% for olodaterol and from 20.5% to 66.6% for indacaterol. This study provides no evidence of a major concern in Europe for olodaterol or indacaterol for off-label use in asthma or for pediatric use.
中文翻译:

丹麦、法国和荷兰没有奥达特罗和茚达特罗超说明书使用的证据:药物利用研究。
描述奥达特罗和茚达特罗在临床实践中的使用特征,并量化哮喘的超说明书使用。荷兰 PHARMO 数据库网络、丹麦人口登记册和法国 IMS 真实世界证据纵向患者数据库面板中 2014 年至 2017 年间奥达特罗或茚达特罗新使用者的药物利用研究。标签上使用的定义是在有慢性阻塞性肺病诊断记录的成年人中使用。超说明书使用被定义为在有哮喘诊断记录但没有慢性阻塞性肺病诊断记录的成人中使用,或在年龄≤18岁的患者中使用。潜在的超说明书使用被定义为没有慢性阻塞性肺病或哮喘的诊断记录。该研究包括 4,158 名奥达特罗新使用者和 9,966 名茚达特罗新使用者。两种药物的超说明书用药发生率均为 3.5%,奥达特罗为 12.4%,茚达特罗为 11.9%。奥达特罗的标签使用率在 47.8% 至 77.7% 之间,茚达特罗的标签使用率在 28.7% 至 70.1% 之间。奥达特罗和茚达特罗的其余新使用者被归类为潜在的超说明书使用者,奥达特罗的患病率为17.3%至48.6%,茚达特罗的患病率为20.5%至66.6%。这项研究没有提供证据表明欧洲对奥达特罗或茚达特罗在哮喘或儿科用途中超说明书使用存在重大担忧。
更新日期:2020-01-17
中文翻译:

丹麦、法国和荷兰没有奥达特罗和茚达特罗超说明书使用的证据:药物利用研究。
描述奥达特罗和茚达特罗在临床实践中的使用特征,并量化哮喘的超说明书使用。荷兰 PHARMO 数据库网络、丹麦人口登记册和法国 IMS 真实世界证据纵向患者数据库面板中 2014 年至 2017 年间奥达特罗或茚达特罗新使用者的药物利用研究。标签上使用的定义是在有慢性阻塞性肺病诊断记录的成年人中使用。超说明书使用被定义为在有哮喘诊断记录但没有慢性阻塞性肺病诊断记录的成人中使用,或在年龄≤18岁的患者中使用。潜在的超说明书使用被定义为没有慢性阻塞性肺病或哮喘的诊断记录。该研究包括 4,158 名奥达特罗新使用者和 9,966 名茚达特罗新使用者。两种药物的超说明书用药发生率均为 3.5%,奥达特罗为 12.4%,茚达特罗为 11.9%。奥达特罗的标签使用率在 47.8% 至 77.7% 之间,茚达特罗的标签使用率在 28.7% 至 70.1% 之间。奥达特罗和茚达特罗的其余新使用者被归类为潜在的超说明书使用者,奥达特罗的患病率为17.3%至48.6%,茚达特罗的患病率为20.5%至66.6%。这项研究没有提供证据表明欧洲对奥达特罗或茚达特罗在哮喘或儿科用途中超说明书使用存在重大担忧。









































 京公网安备 11010802027423号
京公网安备 11010802027423号