Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activation of RSK by phosphomimetic substitution in the activation loop is prevented by structural constraints.
Scientific Reports ( IF 3.8 ) Pub Date : 2020-01-17 , DOI: 10.1038/s41598-019-56937-3 Desiana Somale 1, 2 , Giovanna Di Nardo 3, 4 , Laura di Blasio 2 , Alberto Puliafito 1, 2 , Marianela Vara-Messler 1, 2 , Giulia Chiaverina 1, 2 , Miriam Palmiero 1, 2 , Valentina Monica 1, 2 , Gianfranco Gilardi 3, 4 , Luca Primo 1, 2 , Paolo Armando Gagliardi 2
Scientific Reports ( IF 3.8 ) Pub Date : 2020-01-17 , DOI: 10.1038/s41598-019-56937-3 Desiana Somale 1, 2 , Giovanna Di Nardo 3, 4 , Laura di Blasio 2 , Alberto Puliafito 1, 2 , Marianela Vara-Messler 1, 2 , Giulia Chiaverina 1, 2 , Miriam Palmiero 1, 2 , Valentina Monica 1, 2 , Gianfranco Gilardi 3, 4 , Luca Primo 1, 2 , Paolo Armando Gagliardi 2
Affiliation
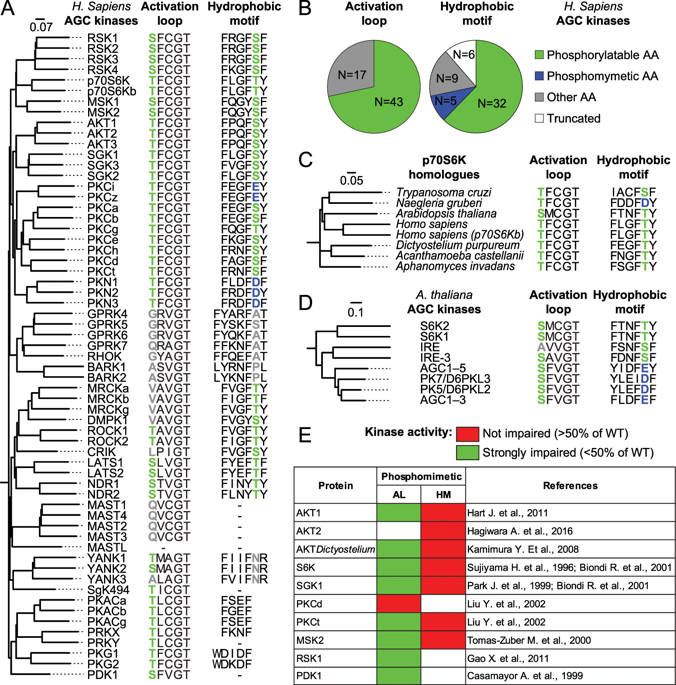
|
The activation of the majority of AGC kinases is regulated by two phosphorylation events on two conserved serine/threonine residues located on the activation loop and on the hydrophobic motif, respectively. In AGC kinase family, phosphomimetic substitutions with aspartate or glutamate, leading to constitutive activation, have frequently occurred at the hydrophobic motif site. On the contrary, phosphomimetic substitutions in the activation loop are absent across the evolution of AGC kinases. This observation is explained by the failure of aspartate and glutamate to mimic phosphorylatable serine/threonine in this regulatory site. By detailed 3D structural simulations of RSK2 and further biochemical evaluation in cells, we show that the phosphomimetic residue on the activation loop fails to form a critical salt bridge with R114, necessary to reorient the αC-helix and to activate the protein. By a phylogenetic analysis, we point at a possible coevolution of a phosphorylatable activation loop and the presence of a conserved positively charged amino acid on the αC-helix. In sum, our analysis leads to the unfeasibility of phosphomimetic substitution in the activation loop of RSK and, at the same time, highlights the peculiar structural role of activation loop phosphorylation.
中文翻译:

通过结构限制来防止在活化环中通过拟磷酸酯取代来活化RSK。
大多数AGC激酶的激活受分别位于激活环和疏水基序上两个保守的丝氨酸/苏氨酸残基上的两个磷酸化事件调节。在AGC激酶家族中,导致组成性活化的天冬氨酸或谷氨酸的拟磷酸酯取代经常发生在疏水基序位点。相反,在整个AGC激酶的进化过程中,激活环中不存在拟磷酸化取代。天冬氨酸和谷氨酸在该调控位点不能模仿可磷酸化丝氨酸/苏氨酸的现象解释了这一观察结果。通过RSK2的详细3D结构模拟和细胞中的进一步生化评估,我们显示了激活环上的拟磷酸化残基未能与R114形成关键的盐桥,是重新排列αC螺旋和激活蛋白质所必需的。通过系统发育分析,我们指出了可磷酸化激活环的可能共同进化以及αC螺旋上存在保守的带正电荷的氨基酸。总而言之,我们的分析导致在RSK的激活环中进行仿生取代的不可行性,同时强调了激活环磷酸化的特殊结构作用。
更新日期:2020-01-17
中文翻译:

通过结构限制来防止在活化环中通过拟磷酸酯取代来活化RSK。
大多数AGC激酶的激活受分别位于激活环和疏水基序上两个保守的丝氨酸/苏氨酸残基上的两个磷酸化事件调节。在AGC激酶家族中,导致组成性活化的天冬氨酸或谷氨酸的拟磷酸酯取代经常发生在疏水基序位点。相反,在整个AGC激酶的进化过程中,激活环中不存在拟磷酸化取代。天冬氨酸和谷氨酸在该调控位点不能模仿可磷酸化丝氨酸/苏氨酸的现象解释了这一观察结果。通过RSK2的详细3D结构模拟和细胞中的进一步生化评估,我们显示了激活环上的拟磷酸化残基未能与R114形成关键的盐桥,是重新排列αC螺旋和激活蛋白质所必需的。通过系统发育分析,我们指出了可磷酸化激活环的可能共同进化以及αC螺旋上存在保守的带正电荷的氨基酸。总而言之,我们的分析导致在RSK的激活环中进行仿生取代的不可行性,同时强调了激活环磷酸化的特殊结构作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号