Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Human antibodies neutralizing diphtheria toxin in vitro and in vivo.
Scientific Reports ( IF 3.8 ) Pub Date : 2020-01-17 , DOI: 10.1038/s41598-019-57103-5 Esther Veronika Wenzel 1 , Margarita Bosnak 1 , Robert Tierney 2 , Maren Schubert 1 , Jeffrey Brown 3 , Stefan Dübel 1 , Androulla Efstratiou 4 , Dorothea Sesardic 2 , Paul Stickings 2 , Michael Hust 1
Scientific Reports ( IF 3.8 ) Pub Date : 2020-01-17 , DOI: 10.1038/s41598-019-57103-5 Esther Veronika Wenzel 1 , Margarita Bosnak 1 , Robert Tierney 2 , Maren Schubert 1 , Jeffrey Brown 3 , Stefan Dübel 1 , Androulla Efstratiou 4 , Dorothea Sesardic 2 , Paul Stickings 2 , Michael Hust 1
Affiliation
|
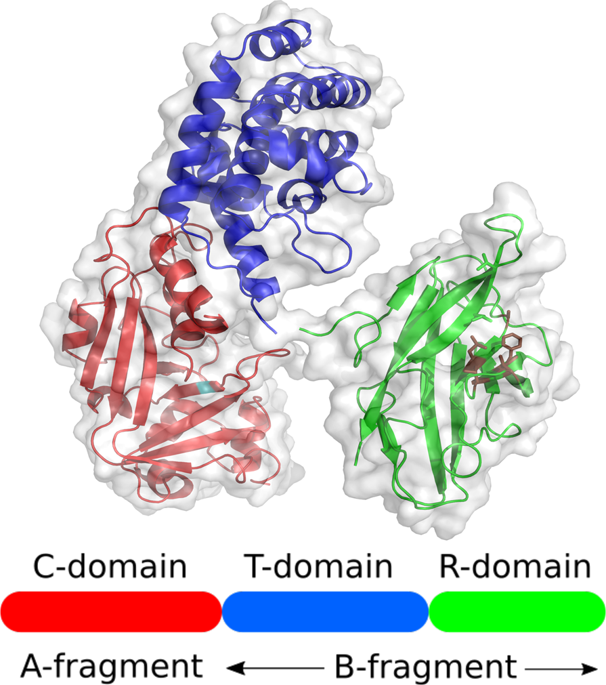
Diphtheria is an infectious disease caused by Corynebacterium diphtheriae. The bacterium primarily infects the throat and upper airways and the produced diphtheria toxin (DT), which binds to the elongation factor 2 and blocks protein synthesis, can spread through the bloodstream and affect organs, such as the heart and kidneys. For more than 125 years, the therapy against diphtheria has been based on polyclonal horse sera directed against DT (diphtheria antitoxin; DAT). Animal sera have many disadvantages including serum sickness, batch-to-batch variation in quality and the use of animals for production. In this work, 400 human recombinant antibodies were generated against DT from two different phage display panning strategies using a human immune library. A panning in microtiter plates resulted in 22 unique in vitro neutralizing antibodies and a panning in solution combined with a functional neutralization screening resulted in 268 in vitro neutralizing antibodies. 61 unique antibodies were further characterized as scFv-Fc with 35 produced as fully human IgG1. The best in vitro neutralizing antibody showed an estimated relative potency of 454 IU/mg and minimal effective dose 50% (MED50%) of 3.0 pM at a constant amount of DT (4x minimal cytopathic dose) in the IgG format. The targeted domains of the 35 antibodies were analyzed by immunoblot and by epitope mapping using phage display. All three DT domains (enzymatic domain, translocation domain and receptor binding domain) are targets for neutralizing antibodies. When toxin neutralization assays were performed at higher toxin dose levels, the neutralizing capacity of individual antibodies was markedly reduced but this was largely compensated for by using two or more antibodies in combination, resulting in a potency of 79.4 IU/mg in the in vivo intradermal challenge assay. These recombinant antibody combinations are candidates for further clinical and regulatory development to replace equine DAT.
中文翻译:

人抗体在体外和体内中和白喉毒素。
白喉是由白喉棒状杆菌引起的传染病。该细菌主要感染喉咙和上呼吸道,产生的白喉毒素(DT)与伸长因子2结合并阻止蛋白质合成,可通过血流传播并影响器官,例如心脏和肾脏。125多年来,针对白喉的治疗一直基于针对DT(白喉抗毒素; DAT)的多克隆马血清。动物血清具有许多缺点,包括血清病,质量之间的批次差异以及将动物用于生产。在这项工作中,使用人类免疫文库从两种不同的噬菌体展示淘选策略中生成了400种针对DT的人类重组抗体。在微量滴定板中淘选产生22种独特的体外中和抗体,在溶液中淘选结合功能中和筛选得到268种体外中和抗体。61种独特的抗体被进一步表征为scFv-Fc,其中35种产生为完全人IgG1。最佳体外中和抗体显示恒定剂量的DT(4x最小细胞病变剂量)IgG形式时,估计相对效力为454 IU / mg,最小有效剂量为50%(MED50%)的3.0 pM。通过免疫印迹和使用噬菌体展示的表位作图分析了35种抗体的靶向结构域。所有三个DT域(酶域,易位域和受体结合域)都是中和抗体的靶标。在较高的毒素剂量水平下进行毒素中和测定时,单个抗体的中和能力显着降低,但是通过组合使用两种或更多种抗体在很大程度上弥补了这种不足,从而在体内皮内激发试验中产生了79.4 IU / mg的效价。这些重组抗体组合是替代马DAT进行进一步临床和法规开发的候选药物。
更新日期:2020-01-17
中文翻译:

人抗体在体外和体内中和白喉毒素。
白喉是由白喉棒状杆菌引起的传染病。该细菌主要感染喉咙和上呼吸道,产生的白喉毒素(DT)与伸长因子2结合并阻止蛋白质合成,可通过血流传播并影响器官,例如心脏和肾脏。125多年来,针对白喉的治疗一直基于针对DT(白喉抗毒素; DAT)的多克隆马血清。动物血清具有许多缺点,包括血清病,质量之间的批次差异以及将动物用于生产。在这项工作中,使用人类免疫文库从两种不同的噬菌体展示淘选策略中生成了400种针对DT的人类重组抗体。在微量滴定板中淘选产生22种独特的体外中和抗体,在溶液中淘选结合功能中和筛选得到268种体外中和抗体。61种独特的抗体被进一步表征为scFv-Fc,其中35种产生为完全人IgG1。最佳体外中和抗体显示恒定剂量的DT(4x最小细胞病变剂量)IgG形式时,估计相对效力为454 IU / mg,最小有效剂量为50%(MED50%)的3.0 pM。通过免疫印迹和使用噬菌体展示的表位作图分析了35种抗体的靶向结构域。所有三个DT域(酶域,易位域和受体结合域)都是中和抗体的靶标。在较高的毒素剂量水平下进行毒素中和测定时,单个抗体的中和能力显着降低,但是通过组合使用两种或更多种抗体在很大程度上弥补了这种不足,从而在体内皮内激发试验中产生了79.4 IU / mg的效价。这些重组抗体组合是替代马DAT进行进一步临床和法规开发的候选药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号