当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Divergent Mechanisms of the Banert Cascade with Propargyl Azides.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.joc.9b03061 Juliana R Alexander 1 , Mary H Packard 1 , Alanna M Hildebrandt 1 , Amy A Ott 1 , Joseph J Topczewski 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.joc.9b03061 Juliana R Alexander 1 , Mary H Packard 1 , Alanna M Hildebrandt 1 , Amy A Ott 1 , Joseph J Topczewski 1
Affiliation
|
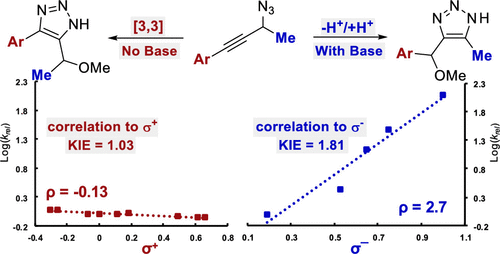
Triazoles are privileged heterocycles for a variety of applications. The synthesis of 1H-triazoles can be accomplished by the Banert cascade from propargylic azides. Depending on the substrate and conditions, the Banert cascade can proceed by either a sigmatropic or prototropic mechanism. This report describes the first detailed kinetic analysis of the Banert cascade proceeding by both pathways including substituent effects and KIE. The analysis identified the inflection point in the divergent pathways, allowing future work to predict which Banert products are accessible.
中文翻译:

Banert级联与炔丙基叠氮化物的发散机理。
三唑是用于各种应用的特权杂环。1H-三唑的合成可以通过炔丙基叠氮化物的Banert级联反应完成。取决于底物和条件,Banert级联反应可以通过σ或质子传递机理进行。该报告描述了包括取代基效应和KIE在内的两种途径对Banert级联反应的首次详细动力学分析。该分析确定了不同途径中的拐点,从而使将来的工作可以预测哪些Banert产品可以使用。
更新日期:2020-01-24
中文翻译:

Banert级联与炔丙基叠氮化物的发散机理。
三唑是用于各种应用的特权杂环。1H-三唑的合成可以通过炔丙基叠氮化物的Banert级联反应完成。取决于底物和条件,Banert级联反应可以通过σ或质子传递机理进行。该报告描述了包括取代基效应和KIE在内的两种途径对Banert级联反应的首次详细动力学分析。该分析确定了不同途径中的拐点,从而使将来的工作可以预测哪些Banert产品可以使用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号